Cory:
Unlock Your AI Assistant Now!
Abstract
Background: Incomplete expansion of balloon-expandable (BE) transcatheter heart valves (THVs) is sometimes treated by ad hoc post-dilatation with an overfilled or larger valvuloplasty balloon. The efficacy of this approach has not been rigorously evaluated, although increased risk for adverse events has been demonstrated. Observational experience suggests that post-dilatation using the original delivery system balloon at the identical filling volume (i.e., double-tap) may routinely improve the degree of THV expansion with low risk.
Aims: We sought to assess the safety and efficacy of a strategy of routine double-tap after BE transcatheter aortic valve implantation (TAVI).
Methods: Patients undergoing TAVI with the SAPIEN 3 Ultra (S3U) valve were prospectively included. Patients with severe annular or subannular calcification were excluded. A validated method of fluoroscopic analysis was utilised to assess the cross-sectional area at the inflow, midpoint, and outflow of the THV before and after double-tap. Thirty-day clinical outcomes were documented.
Results: Routine double-tap was performed in 102 patients. Despite nominal deployment, all patients had some degree of THV underexpansion after the first inflation. Fluoroscopic analysis documented an increase in minimal THV expansion by cross-sectional area of 9.8% for the 20 mm S3U (p=0.151), 9.9% for the 23 mm S3U (p<0.001), 9.2% for the 26 mm S3U (p<0.001), and 8.6% for the 29 mm S3U (p=0.002). There was no stroke or cardiovascular mortality at 30 days.
Conclusions: In favourable anatomy, routine double-tap after BE TAVI improved THV expansion with no safety concerns. The impact of this strategy on THV function, haemodynamic profile, and durability remains to be determined.
Transcatheter aortic valve implantation (TAVI) has become the favoured treatment strategy for most patients with severe aortic stenosis (AS)1. An increase in TAVI utilisation in younger patients with potential for longevity creates a greater need for optimised transcatheter heart valve (THV) function and durability. THV underexpansion following balloon-expandable (BE) TAVI is common2 and has been linked to impaired THV function and durability due to suboptimal leaflet coaptation, pinwheeling, high leaflet stress, alteration in blood flow, hypoattenuated leaflet thickening (HALT), and thrombus2345. Post-dilatation of underexpanded BE THVs can improve expansion, increase the effective orifice area (EOA), decrease the risk of patient-prosthesis mismatch, and reduce paravalvular leak (PVL)6789. Post-dilatation has typically been performed by overfilling the delivery system balloon, using a less compliant balloon, or by using a larger balloon, with a possible increased risk of stroke or annular injury678. The double-tap technique refers to post-dilatation by reusing the delivery system balloon at the same volume10. While ad hoc double-tap has become common in many centres, there has been no systematic evaluation of the safety and efficacy of its routine use. We hypothesised that routine double-tap would improve THV expansion without increasing the risk of adverse procedural events. The present study reports a proof of concept of double-tap on the bench as well as the results of a clinical cohort in which this technique was used routinely after BE TAVI.
Methods
Benchtop assessment
Valve deployment & double-tap
A new 26 mm SAPIEN 3 Ultra (S3U) THV (Edwards Lifesciences) was prepared by trained catheterisation laboratory staff for deployment with a standard Commander delivery system (also Edwards Lifesciences). The valve was deployed at nominal volume (23 mL) on the bench. Following assessment and multimodality imaging, the THV was placed back on the delivery balloon and was inflated a second time at nominal volume. Since access to unused transcatheter THVs for bench testing remains challenging because of limited availability and regulatory constraints, only one valve was used for the purpose of this study.
Hydrodynamic assessment
Hydrodynamic testing was performed using the ViVitro Pulse Duplicator system (ViVitro Labs) in compliance with the International Organization for Standardization (ISO) guidelines (ISO 5840-3:2021)11. The valve was placed inside a silicone holder (Shore A hardness 40±5), and hydrodynamics (mean gradient [MG] in mmHg, and EOA in cm2) were assessed in saline solution, at 37±2°C, 70 bpm, a mean arterial pressure of 100±2 mmHg, and cardiac output of 5.0±0.1 L/min. Valves were averaged from three replicate data collections consisting of 10 consecutive heart cycles each.
Imaging
Multimodality imaging was performed following initial nominal deployment and after double-tap. Video footage from hydrodynamic testing was captured during testing at standardised height and lighting and was utilised for the assessment of valve pinwheeling. Fluoroscopy of the valve was performed using a clinical Innova IGS 520 C-arm system (GE HealthCare). Microcomputed tomography (micro-CT) was completed using the Nikon XT H 225 ST microfocus X-ray tomography system (Nikon). Valve diameters were determined from micro-CT valve area measurements, assessed from the centre for the frame struts at the level of the valve outflow, midpoint, and inflow. Measurements were completed in triplicate and averaged. The expansion percentage was calculated by comparing the average area of the measured outflow, midpoint, and inflow areas to the nominal area of a 26 mm S3U as per the manufacturer (i.e., 519 mm2 for a 26 mm valve)212. The expansion percentage was also calculated at the midpoint level using the same method. Micro-CT measurements were made using ImageJ software, version 2.14.0/1.54f (National Institutes of Health).
Pinwheeling
Pinwheeling refers to twisting of the leaflet’s free edges resulting from excessive leaflet redundancy. Using images from the videos taken during hydrodynamic testing, the pinwheeling index (PWI; %) was determined by tracing the length of the leaflet’s free edge in diastole and comparing it to the ideal length, as previously performed13. This was conducted on 3 simulated cardiac cycles for each leaflet and then averaged to obtain a final PWI value.
Clinical cohort
Study design and patient population
Patients undergoing TAVI with an S3U or an S3U RESILIA THV (both Edwards Lifesciences) at two valve centres in Vancouver, Canada, between August 2023 and March 2024 were prospectively included in this study. Patients were excluded if they had severe annular or subannular calcification, or if they were undergoing valve-in-valve TAVI, or undergoing TAVI with an under- or overfilled balloon or a non-S3U valve. Patients with baseline atrioventricular conduction abnormalities were not excluded. This study was approved by the local institutional ethics committee (University of British Columbia and Providence Health Care Research Ethics Board).
Double-tap procedure
The size of the THV was chosen based on the patient’s native annular area measured by CT and according to the manufacturer’s sizing recommendations. Nominal volume inflation was identified as per the manufacturer’s specifications (11 mL for the 20 mm S3U, 17 mL for the 23 mm S3U, 23 mL for the 26 mm S3U, and 33 mL for the 29 mm S3U THV). Predilatation prior to THV implantation was left to the operator’s discretion. The THV was deployed under rapid pacing, and the balloon was held inflated for 3 seconds with the barrel of the inflation device completely empty in order to ensure complete inflation of the balloon. The THV delivery system was then retracted and parked in the descending aorta. The THV delivery balloon was subsequently readvanced and fully inflated at nominal volume for 3 seconds during rapid pacing. Cerebral protection devices were not utilised.
Fluoroscopic image acquisition and analysis
Assessment of THV expansion was performed using a recently described fluoroscopic method using the commissural post height of the S3U for calibration14. The commissural post height of the S3U varies according to the THV size but is fixed for a defined THV size and remains unchanged independently of THV expansion. This method showed high interobserver agreement and excellent correlation with CT-assessed expansion. In brief, non-contrast cine images (15 frames per second) were obtained immediately after THV deployment using standard catheterisation laboratory equipment (GE Innova 520 [GE HealthCare]) in the three-cusp (3C) and cusp-overlap (CO) views. The images were obtained with the THV stent frame aligned to minimise parallax. This resulted in minor modifications to the predetermined CT angles, which were then referred to as the fluoroscopically corrected 3C and CO views. After double-tap, non-contrast cine images at the same frame speed and magnification were obtained again using the corrected 3C and CO views. The THV diameter (mm) at the inflow, midpoint, and outflow was measured in both the 3C and CO views before and after double-tap. The THV cross-sectional area was derived from the diameter at each level using basic mathematics. Percentage expansion was calculated using the cross-sectional area derived from the average diameter in the 3C and CO views compared to the nominal area as per the manufacturer (e.g., 328 mm2 for the 20 mm S3U, 409 mm2 for the 23 mm S3U, 519 mm2 for the 26 mm S3U, and 649 mm2 for the 29 mm S3U). Percentage expansion was calculated before and after double-tap. syngo Dynamics imaging (Siemens Healthineers) and TOMTEC software (TOMTEC Imaging Systems) were used for image analysis.
Endpoints
The primary endpoint was the efficacy of routine double-tap on THV expansion. The primary safety endpoint was the incidence of major adverse outcomes according to the Valve Academic Research Consortium (VARC)-3 criteria15. Safety outcomes included death, myocardial infarction, stroke, annular injury, aortic dissection, and permanent pacemaker implantation. THV haemodynamic outcomes, including aortic valve gradients, maximum velocity (Vmax), area, and paravalvular regurgitation by transthoracic echocardiography, were collected. Outcomes were assessed immediately after the procedure and at 30-day follow-up. Finally, the impacts of the degree of oversizing (%) and aortic valve calcification (Agatston units) on the effectiveness of double-tap were assessed.
Statistical analysis
Continuous variables are presented as mean with standard deviation or median with interquartile range (IQR), as appropriate. Categorical variables are presented as counts and percentages. The normality of distribution for all variables was assessed using the Shapiro-Wilk test. Comparisons between variables were performed using the Student’s t-test, Mann-Whitney U test, chi-square (χ²) test, or Fisher’s exact test, as appropriate. To assess predictors of double-tap, we performed linear regression using oversizing degree and aortic valve calcium burden as continuous independent variables, with double-tap efficacy (expansion gain) as the dependent variable. Additionally, we conducted a logistic regression analysis using a binary outcome defined as above- versus below-median expansion improvement. Odds ratios (ORs) with 95% confidence intervals (CIs) were reported. Statistical analysis was performed utilising jamovi, version 2.5 (Computer Software), with a statistical significance threshold set at p<0.05.
Results
Double-tap: benchtop results
Micro-CT demonstrated improved expansion of the 26 mm S3U valve after double-tap (Figure 1A). After nominal inflation, the THV was underexpanded to various degrees at the inflow, midpoint, and outflow. The midpoint showed the smallest diameter, measuring 24.0 mm. The THV expansion by area at the midpoint was 87.2% after the first inflation. Following double-tap, the THV expansion by area at the midpoint improved to 93.8%. The impact of double-tap was also observed on fluoroscopy with visually improved and more symmetrical expansion (Figure 1B). Hydrodynamic assessment showed similar results in terms of MG and EOA between initial deployment and following double-tap (Figure 1C). However, there was a clear improvement in terms of pinwheeling following double-tap, with the PWI dropping from 14.0% to 4.6%, as observed during hydrodynamic testing (Moving image 1). 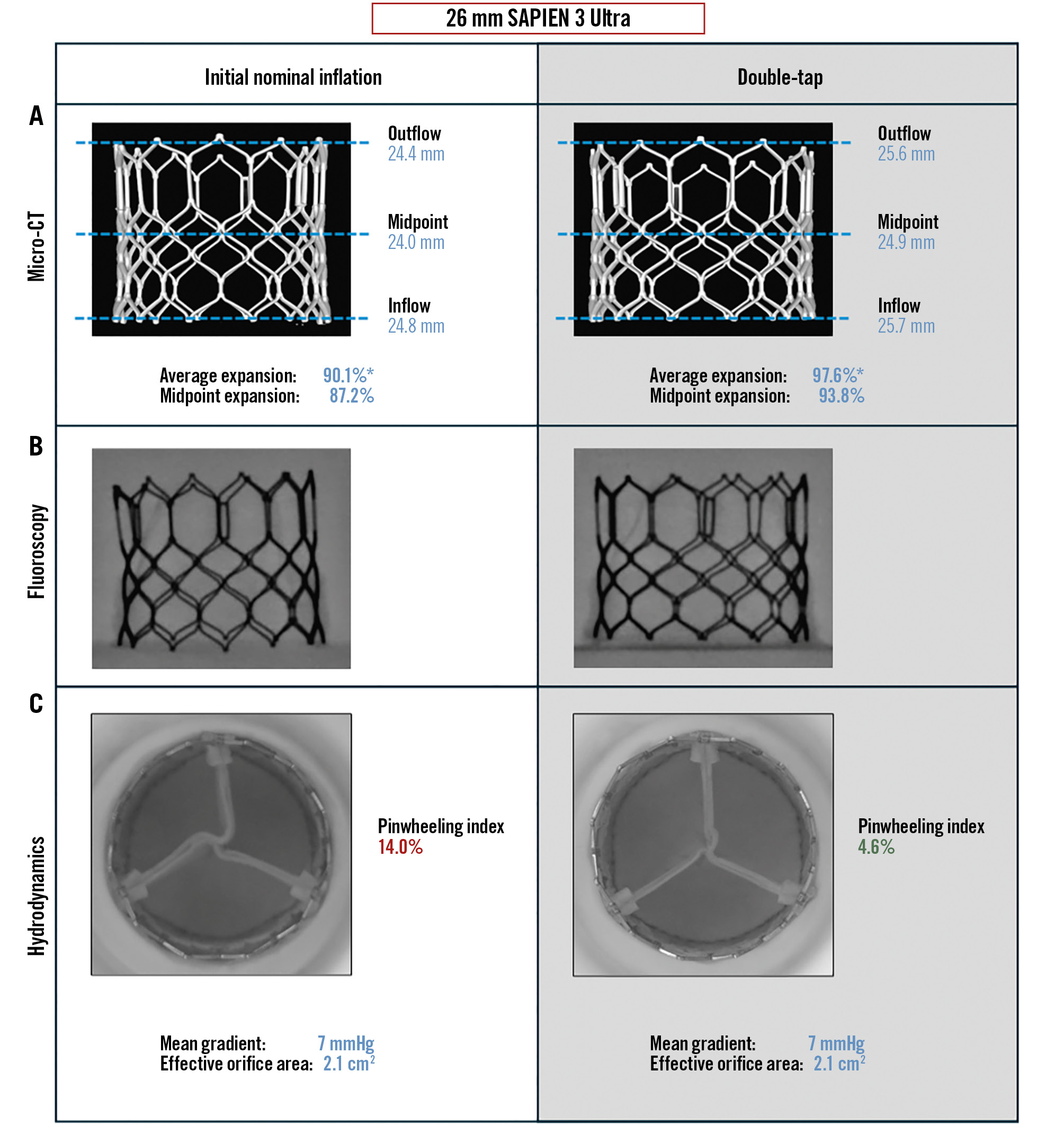
Figure 1. Bench assessment of the impact of double-tap on a 26 mm SAPIEN 3 Ultra. Comparison after initial deployment (left column) and following double-tap (right column). THV expansion on micro-CT (A), on fluoroscopy (B), and hydrodynamic function (C). *Average expansion combining the inflow, midpoint, and outflow areas. Micro-CT: microcomputed tomography; THV: transcatheter heart valve
Study population and baseline characteristics
In total, 102 patients who underwent TAVI with the S3U or S3U RESILIA valve and met the inclusion criteria were included in this study. Baseline characteristics are presented in Table 1. The mean age was 78.8±7.8 years, and 36% were female. The left ventricular ejection fraction and aortic valve mean gradient were 58.7±10.1% and 43.7±12.0 mmHg, respectively. Seven (7%) patients had a bicuspid aortic valve. A prior pacemaker was present in 8 (8%) patients, and 8 (8%) had baseline right bundle branch block prior to TAVI.
Table 1. Baseline characteristics of patients.
| Characteristics | All patients (n=102) |
|---|---|
| BMI, kg/m2 | 26.9±6.1 |
| Age, years | 78.8±7.8 |
| Male | 65 (64) |
| Bicuspid aortic valve | 7 (7) |
| LFLG-AS | 8 (8) |
| Paradoxical LFLG-AS | 9 (9) |
| LVEF, % | 58.7±10.1 |
| MG, mmHg | 43.7±12.0 |
| Underlying heart disease | |
| CAD with prior PCI | 26 (25) |
| Prior CABG | 11 (11) |
| Prior SAVR | 0 (0) |
| Prior TAVI | 0 (0) |
| Comorbidities | |
| Anaemia | 23 (23) |
| Chronic renal failure | 36 (35) |
| Haemodialysis | 2 (2) |
| Peripheral artery disease | 8 (8) |
| COPD | 18 (18) |
| Hypertension | 68 (67) |
| Diabetes mellitus | 27 (26) |
| Atrial fibrillation | 19 (19) |
| Prior stroke/TIA | 14 (14) |
| Prior pacemaker | 8 (8) |
| RBBB | 8 (8) |
| LBBB | 11 (11) |
| Data are mean±standard deviation or n (%). BMI: body mass index; CABG: coronary artery bypass grafting; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; LBBB: left bundle branch block; LFLG-AS: low-flow, low-gradient aortic stenosis; LVEF: left ventricular ejection fraction; MG: mean gradient; PCI: percutaneous coronary intervention; RBBB: right bundle branch block; SAVR: surgical aortic valve replacement; TAVI: transcatheter aortic valve implantation; TIA: transient ischaemic attack | |
Procedural characteristics
Procedural characteristics are presented in Table 2. The most common THV sizes used in the study were the 26 mm S3U (n=49, 48%) and the 23 mm S3U (n=42, 41%), followed by the 29 mm S3U (n=7, 7%) and the 20 mm S3U (n=4, 4%). Fifty (49%) patients had mild or moderate annular and/or subannular calcification. Predilatation was only performed in 10 (9.8%) patients.
Table 2. Procedural characteristics of patients.
| Characteristics | |
|---|---|
| Pre-TAVI CT | |
| Annular/subannular calcification | |
| None | 52 (51) |
| Mild | 42 (41) |
| Moderate | 8 (8) |
| Severe | 0 (0) |
| Aortic valve dimensions | |
| Minimum diameter, mm | 22.2 |
| Maximum diameter, mm | 26.8 |
| Mean annular diameter, mm | 24.0 |
| Mean annular area, mm2 | 462.9 |
| THV type implanted | |
| S3 Ultra | 75 (74) |
| S3 Ultra RESILIA | 27 (26) |
| THV size implanted | |
| 20 mm | 4 (4) |
| 23 mm | 42 (41) |
| 26 mm | 49 (48) |
| 29 mm | 7 (7) |
| Procedural characteristics | |
| Access | |
| Transfemoral | 101 (99) |
| Subclavian | 1 (1) |
| Predilatation | 10 (10) |
| THV implantation at nominal volume | 102 (100) |
| THV post-dilatation at nominal volume | 102 (100) |
| Data are n or n (%). CT: computed tomography; S3: SAPIEN 3; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve | |
Effect of double-tap on THV expansion
Table 3, Table 4, Figure 2, and the Central illustration report the effect of double-tap on THV diameter and area by expansion across all THV sizes. Underexpansion was most prominent at the midpoint of the THV. Hence, the diameter/area measured at the midpoint was referred to as the minimal THV diameter/area.
Table 3. Effect of double-tap on THV diameter.
| THV size and level | Before double-tap: diameter, mm | After double-tap: diameter, mm | Absolute increase in diameter, mm | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| 3C view | CO view | 3C view | CO view | 3C view | CO view | 3C view | CO view | |
| 20 mm S3U (n=4) | ||||||||
| Inflow | 18.5±1.2 | 18.6±1.0 | 19.4±0.9 | 19.2±1.0 | 0.9±0.3 | 0.6±0.7 | 0.281 | 0.396 |
| Midpoint* | 17.0±1.4 | 17.3±1.0 | 18.4±1.2 | 18.5±1.3 | 1.4±0.3 | 1.3±0.6 | 0.171 | 0.166 |
| Outflow | 18.1±1.1 | 18.5±1.0 | 19.3±1.0 | 19.2±1.1 | 1.1±0.4 | 0.7±0.9 | 0.178 | 0.406 |
| 23 mm S3U (n=42) | ||||||||
| Inflow | 21.8±1.1 | 21.3±0.9 | 22.6±1.0 | 22.1±0.9 | 0.8±0.7 | 0.8±0.7 | <0.001 | <0.001 |
| Midpoint* | 20.7±1.1 | 20.3±1.0 | 22.0±1.1 | 21.5±1.0 | 1.3±0.7 | 1.2±0.7 | <0.001 | <0.001 |
| Outflow | 22.1±0.9 | 21.8±0.9 | 23.0±1.0 | 22.7±0.9 | 0.9±0.7 | 0.9±0.6 | <0.001 | <0.001 |
| 26 mm S3U (n=49) | ||||||||
| Inflow | 24.5±1.0 | 24.0±1.0 | 25.2±1.0 | 24.9±0.9 | 0.7±0.5 | 0.9±0.7 | <0.001 | <0.001 |
| Midpoint* | 23.1±1.1 | 22.8±1.1 | 24.4±1.0 | 24.1±1.0 | 1.3±0.6 | 1.3±0.8 | <0.001 | <0.001 |
| Outflow | 24.6±0.8 | 24.4±1.0 | 25.4±0.9 | 25.3±1.0 | 0.8±0.6 | 0.9±0.6 | <0.001 | <0.001 |
| 29 mm S3U (n=7) | ||||||||
| Inflow | 27.1±0.8 | 26.7±0.8 | 28.2±0.5 | 27.6±0.7 | 1.1±0.7 | 0.9±0.6 | 0.014 | 0.066 |
| Midpoint* | 25.8±0.5 | 25.7±1.0 | 27.2±0.5 | 27.0±0.8 | 1.4±0.6 | 1.3±0.7 | <0.001 | 0.039 |
| Outflow | 27.3±0.8 | 27.6±0.5 | 28.4±0.6 | 28.4±0.6 | 1.1±0.6 | 0.8±0.4 | 0.017 | 0.030 |
| Data are mean±standard deviation. *Minimal THV diameter. 3C: three-cusp; CO: cusp-overlap; S3U: SAPIEN 3 Ultra; THV: transcatheter heart valve | ||||||||
Table 4. Effect of double-tap on the minimal THV expansion by area.
| THV size | Before double-tap: expansion, % | After double-tap: expansion, % | Increase in minimal THV expansion, % | p-value |
|---|---|---|---|---|
| 20 mm S3U (n=4) | 70.2±8.9 | 80.0±10.8 | 9.8 | 0.151 |
| 23 mm S3U (n=42) | 81.0±7.6 | 90.9±8.2 | 9.9 | <0.001 |
| 26 mm S3U (n=49) | 80.4±6.4 | 89.6±5.7 | 9.2 | <0.001 |
| 29 mm S3U (n=7) | 80.6±3.6 | 89.2±2.8 | 8.6 | 0.002 |
| Data are % or %±standard deviation.*The minimal THV area corresponds to the midpoint of the THV frame and was calculated based on the average area of the 3C and CO views. 3C: three-cusp; CO: cusp-overlap; S3U: SAPIEN 3 Ultra; THV: transcatheter heart valve | ||||
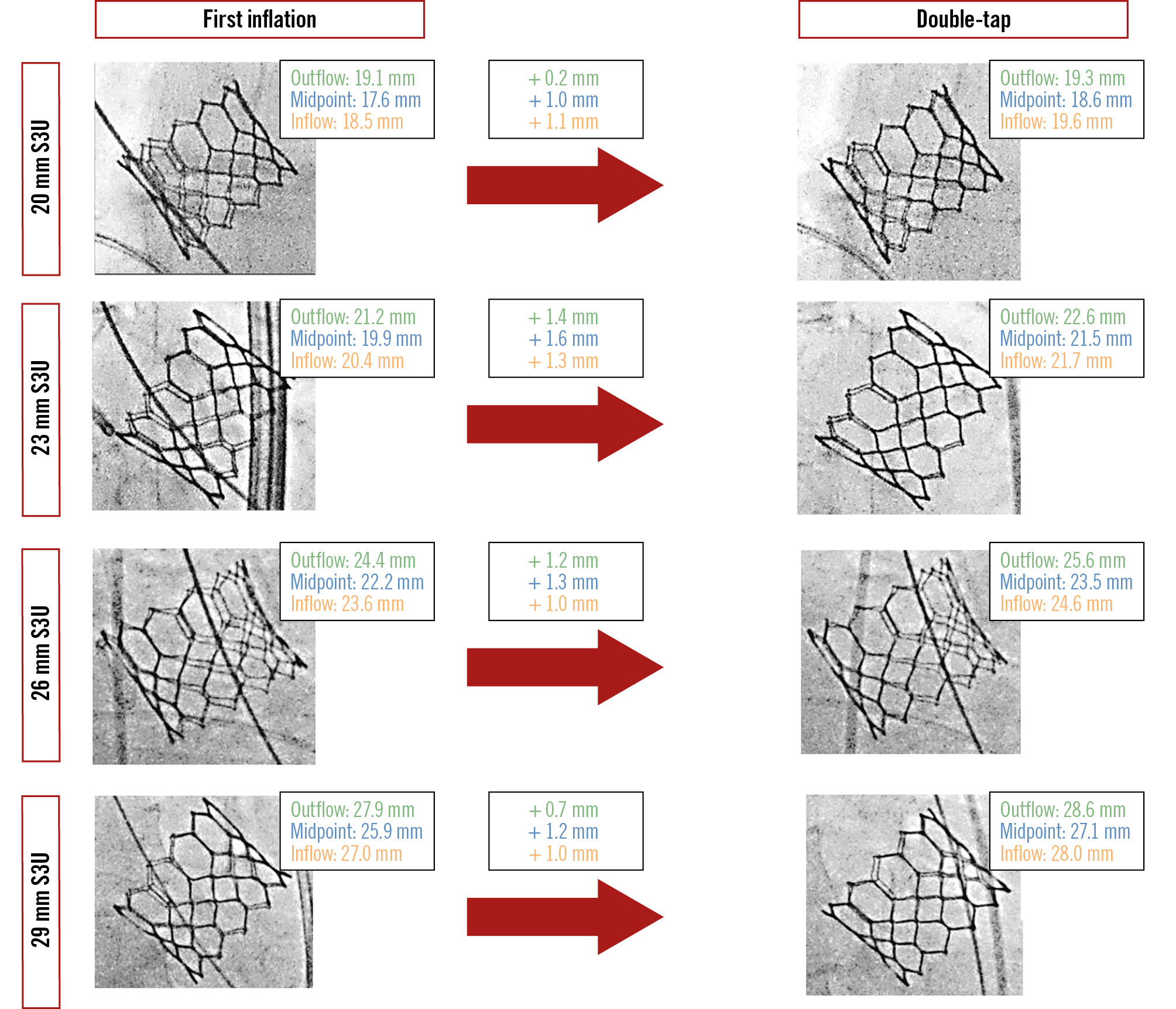
Figure 2. Examples of the effect of double-tap on valve expansion according to THV size. S3U: SAPIEN 3 Ultra; THV: transcatheter heart valve.
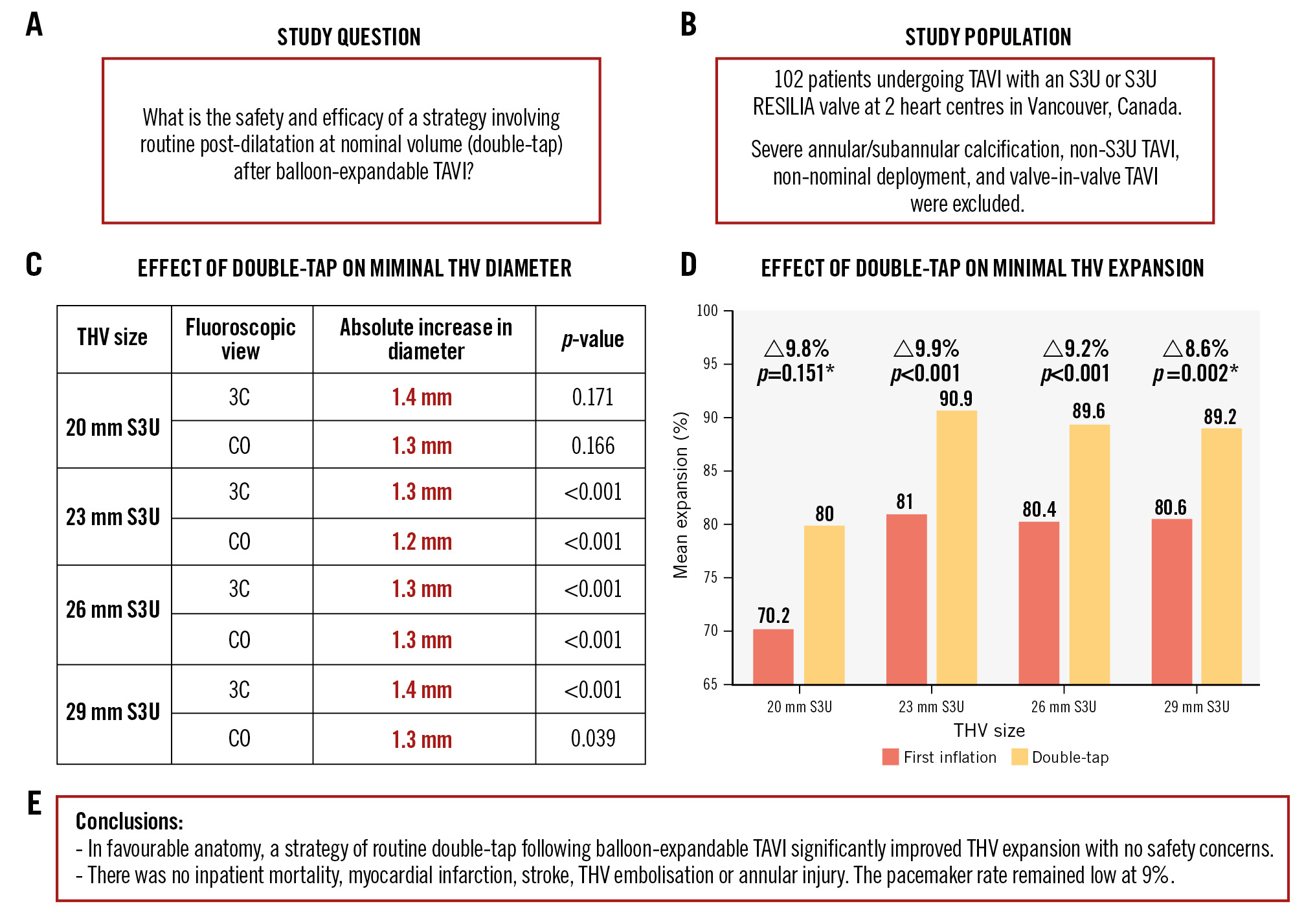
Central illustration. Routine post-dilatation at nominal volume after balloon-expandable TAVI. A) Study question. B) Study population. Effects of double-tap on minimal THV diameter (C) and expansion (D). E) Study conclusions. *small (<10 patients) subgroup. 3C: three-cusp; CO: cusp-overlap; S3U: SAPIEN 3 Ultra; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve
20 mm S3U (n=4)
In the 3C view, the minimal THV diameter for the 20 mm S3U was 17.0±1.4 mm after the first inflation and 18.4±1.2 mm after double-tap (absolute increase in diameter: 1.4±0.3 mm; p=0.171). In the CO view, the minimal THV diameter was 17.3±1.0 mm after the first inflation and 18.5±1.3 mm after double-tap (absolute increase in diameter: 1.3±0.6 mm; p=0.166). The minimal THV expansion was 70.2±8.9% after the first inflation and 80.0±10.8% after double-tap (absolute increase in expansion: 9.8%; p=0.151).
23 mm S3U (n=42)
The minimal THV diameter for the 23 mm S3U was 20.7±1.1 mm after the first inflation in the 3C view and 22.0±1.1 mm after double-tap (absolute increase in diameter: 1.3±0.7 mm; p<0.001). In the CO view, the minimal THV diameter was 20.3±1.0 mm after the first inflation and 21.5±1.0 mm after double-tap (absolute increase in diameter: 1.2±0.7 mm; p<0.001). The minimal THV expansion was 81.0±7.6% after the first inflation and 90.9±8.2% after double-tap (absolute increase in expansion: 9.9%; p<0.001).
26 mm S3U (n=49)
The minimal THV diameter for the 26 mm S3U was 23.1±1.1 mm after the first inflation in the 3C view and 24.4±1.0 mm after double-tap (absolute increase in diameter: 1.3±0.6 mm; p<0.001). In the CO view, the minimal THV diameter was 22.8±1.1 mm after the first inflation, and 24.1±1.0 mm after double-tap (absolute increase in diameter: 1.3±0.8 mm; p<0.001). The minimal THV expansion was 80.4±6.4% after the first inflation and 89.6±5.7% after double-tap (absolute increase in expansion: 9.2%; p<0.001).
29 mm S3U (n=7)
The minimal THV diameter for the 29 mm S3U was 25.8±0.5 mm after the first inflation in the 3C view and 27.2±0.5 mm after double-tap (absolute increase in diameter: 1.4±0.6 mm; p<0.001). In the CO view, the minimal THV diameter was 25.7±1.0 mm after the first inflation and 27.0±0.8 mm after double-tap (absolute increase in diameter: 1.3±0.7 mm; p=0.039). The minimal THV expansion was 80.6±3.6% after the first inflation and 89.2±2.8% after double-tap (absolute increase in expansion: 8.6%; p=0.002).
Clinical and safety endpoints
Table 5 presents the inpatient and 30-day outcomes of the present cohort. There was no inpatient mortality, stroke, myocardial infarction, annular injury, or THV embolisation. The median length of hospital stay was 1 day, and the mean gradient on echocardiography at discharge was 8.4±3.9 mmHg. At 30 days, there was no cardiovascular mortality or late stroke. Complete or high-degree heart block requiring pacemaker implantation occurred in 8 patients (9%). Six (6%) patients required rehospitalisation at 30 days (4 for heart block and 2 for a non-cardiovascular cause). The transvalvular aortic mean gradient on echocardiography at 30 days was 12.1±4.7 mmHg.
Table 5. Inpatient outcomes, adverse events, and 30-day follow-up.
| Characteristics | All patients (n=102) |
|---|---|
| Inpatient outcomes | |
| All-cause death | 0 (0) |
| Stroke | 0 (0) |
| MI | 0 (0) |
| Major bleeding | 0 (0) |
| Annular injury | 0 (0) |
| Complete heart block* | 4 (4) |
| Access closure complication** | 1 (1) |
| Pericardial effusion | 0 (0) |
| New LBBB | 4 (4) |
| Valve embolisation | 0 (0) |
| Mild PVL | 3 (3) |
| Moderate to severe PVL | 0 (0) |
| Hospital LOS, days | 1 [1-1] |
| MG at discharge, mmHg | 8.4±3.9 |
| 30-day outcomes | |
| Cardiovascular mortality | 0 (0) |
| Non-cardiovascular mortality | 1 (1) |
| Complete/high-degree heart block* | 8 (9) |
| Stroke | 0 (0) |
| Rehospitalisation rate | 6 (6) |
| MG at 30 days, mmHg | 12.1±4.7 |
| Data are n (%), mean±standard deviation, or median [interquartile range]. *Requiring permanent pacemaker implantation. **Failure of closure devices to achieve haemostasis at the arteriotomy site, leading to surgical vascular repair. LBBB: left bundle branch block; LOS: length of stay; MG: mean gradient; MI: myocardial infarction; PVL: paravalvular leak | |
Predictors of double-tap efficacy
In the linear regression model, neither oversizing degree (p=0.95) nor aortic valve calcium burden (p=0.48) showed a significant association with the extent of valve expansion improvement following double-tap. Similarly, in the logistic regression analysis, neither oversizing (OR 1.01, 95% CI: 0.94-1.10; p=0.74) nor calcium burden (OR 1.00, 95% CI: 1.00-1.00; p=0.81) predicted binary double-tap efficacy based on the median expansion gain.
Discussion
To our knowledge, this is the first study assessing the impact of routine post-dilatation with the double-tap technique following TAVI with BE valves. The key findings of this study are as follows: (1) despite nominal deployment of the BE valve, all patients ended up with various degrees of THV underexpansion after the first inflation; (2) routine use of double-tap resulted in a statistically significant improvement in THV expansion, which was observed in both the 3C and CO views and was consistent across the different THV sizes; (3) this strategy appears to be safe; and (4) the impact of double-tap can also be appreciated on the bench, resulting in better valve expansion and function even in an idealised ex vivo setup.
Underexpansion of the SAPIEN 3 Ultra valve
Despite nominal deployment, THV underexpansion was present in all patients after the first inflation, particularly at the midpoint, where the THV is often in direct contact with the largest calcium burden. This finding is in line with recent post-TAVI CT studies of patients undergoing TAVI with the S3U THV, showing that the prevalence of THV deformation and underexpansion can be as high as 92%2, and underexpansion at the midpoint is extremely common even in cases where the THV system balloon is overfilled16. Here, even on the bench and without external constriction, the S3U THV did not achieve nominal expansion (THV midpoint expansion: 87.2%). It can be speculated that underexpansion can be even more prominent when deployed in a restricted and/or calcified environment.
Efficacy of double-tap
The benefit of double-tap was observed to be consistent across all THV sizes. The possible exception was the smallest S3U (20 mm), where the benefit was numerically present but not significant statistically, possibly due to the limited number of patients in this group. Interestingly, double-tap efficacy was not significantly influenced by baseline anatomical factors such as annular oversizing or calcification severity, although the sample size is certainly too small to draw a definitive conclusion on this subanalysis. The underlying mechanism by which double-tap improves THV expansion is unclear but might be explained by a reduction in recoil, focused balloon force on the midpoint of the partially expanded THV, or by a longer inflation duration. Indeed, the concept of double inflation has also been shown to be largely effective in coronary stents where it improved stent minimal lumen area by as much as 14%17. The stent frame of the S3U valve is made of cobalt chromium, which is prone to acute elastic recoil, a concept also well described in the coronary stent literature18. Prior studies have found that elastic recoil is associated with a mean reduction in THV diameter by 3.8% to 4.7%1920. This phenomenon may be mitigated by routine double-tap. On the bench, the impact of the double-tap was only observed on expansion parameters, with no immediate effect on EOA or MG. However, this does not negate other impacts of underexpansion, including increased flow turbulence, the development of HALT, and potential implications for durability242122. This is highlighted by the fact that double-tap improved pinwheeling during hydrodynamic testing. Underexpansion associated with pinwheeling has been shown to lead to leaflet deterioration over time and is thought to accelerate valve deterioration4.
Safety of double-tap
One of the major concerns with post-dilatation during TAVI procedures has been the risk for potential complications, including stroke and annular injury, THV embolisation, and increased pacemaker rate67823. However, post-dilatation is generally performed by adding more volume to the delivery balloon or with larger or non-compliant balloons. Here, double-tap improved THV expansion without the need for adding more volume or using dedicated balloons. This could be of particular interest in patients with adverse root features, as the procedural risks associated with an aggressive post-dilatation strategy can be significant10. Indeed, in the present experience, routine double-tap appeared safe, with no signal suggesting an increased risk for procedural adverse events. There was no death, stroke, annular injury, or valve embolisation, and the rate of complete/high-degree heart block requiring pacemaker implantation was relatively low.
Limitations
Routine double-tap cannot be recommended for patients with severe annular or subannular calcification, as they were excluded from the current study. Intraprocedural CT would have been desirable, but obviously impractical. However, the fluoroscopic commissural post height method has been previously validated and compared against CT14. It must also be noted that predilatation was performed only in a limited number of cases, and whether its use influences the efficacy of double-tap remains unknown. Additionally, neither invasive nor non-invasive assessment of THV haemodynamics were performed before or after double-tap. Thus, larger studies are needed to determine whether this improvement in THV expansion will translate into a superior haemodynamic profile, lower incidence of HALT, or better valve durability.
Conclusions
In the absence of adverse root features, routine post-dilatation using the same delivery system balloon at nominal volume after BE TAVI was associated with a substantial and statistically significant improvement in THV expansion with no procedural safety concerns.
Impact on daily practice
When transcatheter heart valve (THV) underexpansion or paravalvular leak is apparent after balloon-expandable (BE) transcatheter aortic valve implantation (TAVI), redilatation with an overfilled or larger balloon may result in visible improvement of the THV stent frame and a reduction in paravalvular leak, at the cost of an increased risk for adverse procedural events. Our study suggests that a strategy of routine post-dilatation using the same delivery system balloon at nominal volume (i.e., double-tap) after BE TAVI results in improved THV expansion with no major safety concerns in patients without adverse root features on computed tomography.
Conflict of interest statement
D.A. Wood is a consultant for and his institution receives unrestricted grant support from Edwards Lifesciences, Medtronic, and Abbott; he has equity in Excision Medical. J.G. Webb is a consultant for Edwards Lifesciences; and receives research funding from Edwards Lifesciences, Medtronic, and Boston Scientific. J.A. Leipsic is supported by a Canadian Research Chair in Advanced Cardiopulmonary Imaging; is a consultant for HeartFlow Inc. and Circle Cardiovascular Imaging; and provides CT core lab services for Edwards Lifesciences, Medtronic, Abbott, and Boston Scientific, for which no direct compensation is received. J. Sathananthan has received speaking fees from Edwards Lifesciences; is a consultant for Edwards Lifesciences, Boston Scientific, NVT Medical, and Medtronic; has received research support from Medtronic, ViVitro Labs, and Edwards Lifesciences; and is also an employee of Boston Scientific. S.L. Sellers is a consultant for Edwards Lifesciences, Anteris, Excision Medical, and Medtronic; has received research support from Medtronic, ViVitro Labs, and Edwards Lifesciences; and has stock options in Excision Medical. J. Ye is a consultant for Edwards Lifesciences. D. Meier has received an institutional grant from Edwards Lifesciences. The other authors have no relevant conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Hydrodynamic testing of the SAPIEN 3 Ultra valve after the first inflation (left) and after double-tap (right).

