Cory:
Unlock Your AI Assistant Now!
Abstract
Background: Evidence supporting the clinical superiority of the Watchman FLX with respect to its previous iteration, the Watchman 2.5, is still sparse.
Aims: We aimed to compare the Watchman FLX and Watchman 2.5 in terms of device-related complications and clinical outcomes.
Methods: All consecutive left atrial appendage closures (LAACs) completed with implantation of a Watchman device at two high-volume centres between July 2018 and January 2023 were considered. Based on the type of implanted device, patients were assigned to either the Watchman FLX or Watchman 2.5 group. The study endpoints included device-related thrombus (DRT) and peridevice leak (PDL), as evaluated by transoesophageal echocardiography (TOE), and stroke rate at the longest available follow-up. Propensity score matching (PSM) analysis was used to minimise baseline differences between groups.
Results: After performing PSM, 1,128 patients were included in each group. In the TOE follow-up, performed at a mean of 2.3 months, both DRT (0.2% vs 3.1%; hazard ratio [HR] 0.35, 95% confidence interval [CI]: 0.21-0.38; p=0.017) and PDL (21.0% vs 30.6%; HR 0.68, 95% CI: 0.59-0.77; p=0.031) were significantly lower in the Watchman FLX group compared with the Watchman 2.5 group. At a mean of 1.6 years of follow-up, the stroke rate was numerically lower in the Watchman FLX group compared with the Watchman 2.5 group (3.4% vs 5.1%; HR 0.56, 95% CI: 0.15-1.69; p=0.078).
Conclusions: In a large dual-centre cohort of consecutive, successful LAAC procedures using two iterations of the Watchman device, the Watchman FLX was associated with significantly lower rates of both DRT and PDL compared to the Watchman 2.5.
Percutaneous left atrial appendage (LAA) closure (LAAC) is an established therapeutic option for preventing stroke in patients with non-valvular atrial fibrillation (AF) and increased bleeding risk. The aim of the procedure is to mechanically exclude from circulation the LAA, which is the main source of cardiac thrombi, without leaving a residual leak or developing thrombus on the atrial surface of the implanted device1.
The Watchman 2.5 (Boston Scientific), which received the European Conformity (CE) mark in 2005 and U.S. Food and Drug Administration (FDA) approval in 20152, has been the most frequently used device for LAAC worldwide. It consists of a self-expanding nitinol cage covered with a porous membrane on the proximal face, secured with 10 fixation barbs located circumferentially. Long-term efficacy and safety have been established in two randomised clinical trials (RCTs) and several large-scale registries345. In 2019, the second-generation Watchman FLX (also Boston Scientific) was released, with design iterations including the shortening of the lobe length, the blunting of the distal part, the addition of a second row of J-shaped barbs, and the introduction of a flat proximal surface. These modifications aimed to improve LAA sealing, reduce both device permeability and thrombus formation risk, and facilitate implantation in complex LAA anatomies678. The two devices have been compared in a few multicentre observation studies, which have suggested the new Watchman FLX might be associated with lower risks of device-related thrombus (DRT) and peridevice leak (PDL) compared with the older-generation device91011. However, because of the limitations of these studies, there is still uncertainty about the degree to which the Watchman FLX has superior results in terms of DRT and residual PDL compared with the Watchman 2.5, and whether it ultimately offers better protection against long-term thromboembolic events.
Against this background, we aimed to compare the Watchman FLX with the Watchman 2.5 in terms of DRT, PDL, and clinical events during long-term follow-up, using a propensity score-matched analysis to minimise baseline differences between groups.
Methods
Study design
The study cohort was retrospectively assembled from two institutional datasets, comprising all consecutive LAAC procedures performed at Bern University Hospital (Bern, Switzerland) and the Texas Cardiac Arrhythmia Institute at St. David’s Medical Center (Austin, TX, USA) between July 2018 and January 2023. All data were prospectively collected in local registries according to prespecified protocols. All patients who received an LAAC device other than the Watchman or patients who refused to participate in the local LAAC registries were excluded by this analysis. The study flowchart is shown in Figure 1.
Demographic and clinical characteristics, information related to LAAC interventions, and follow-up data were prospectively collected. Two experienced cardiologists from each centre evaluated transoesophageal echocardiography (TOE) images for the presence of either PDL around the LAA or DRT on the atrial surface of the device. A third reviewer was involved in case of disagreement between the two experts. The clinical outcomes were locally adjudicated by trained cardiologists, while the strokes were adjudicated by neurologists.
The study complied with the Declaration of Helsinki and with the local ethics committee of each centre.
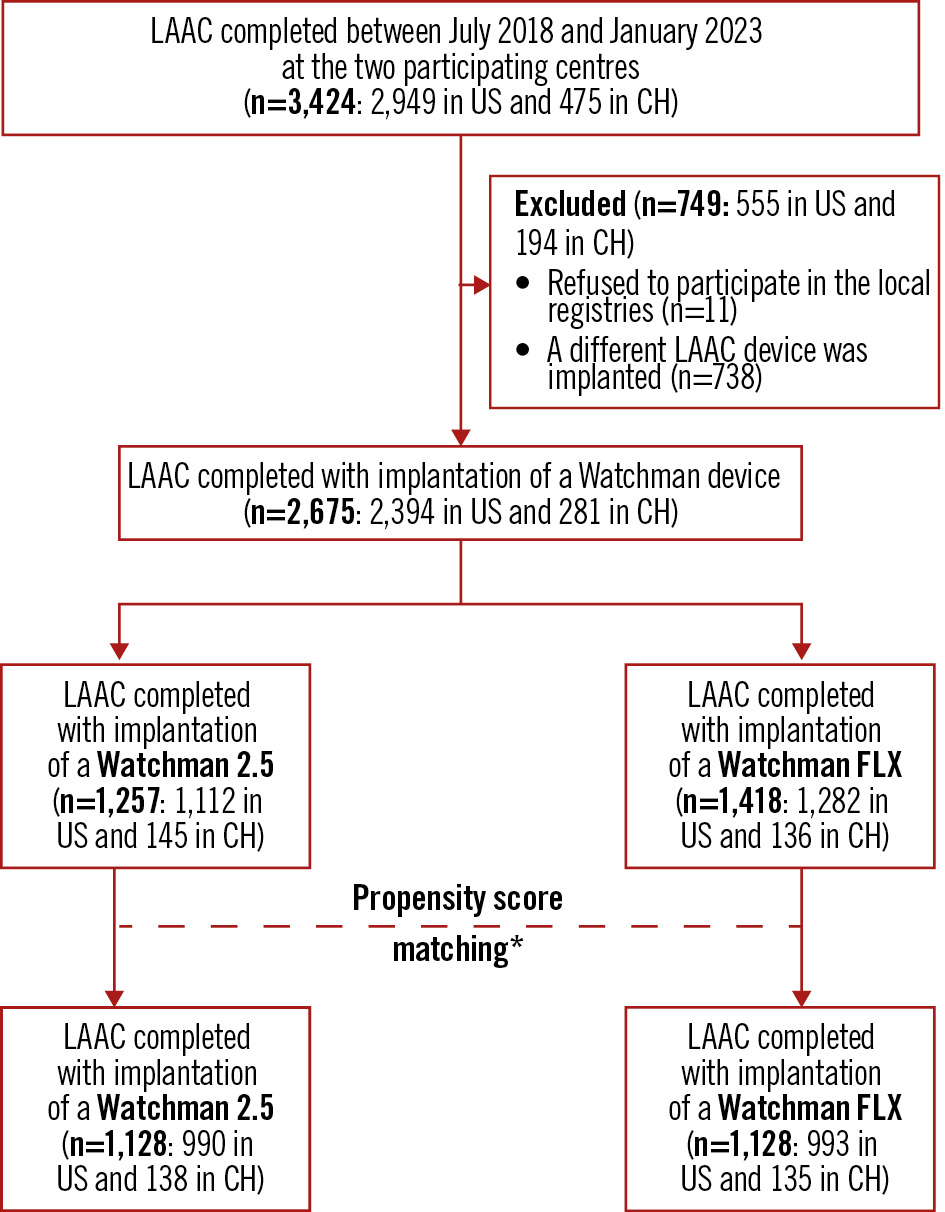
Figure 1. Patient flowchart. *The control group was identified as the Watchman 2.5 cohort by 1:1 propensity score matching with the Watchman FLX group. The scores were estimated using a non-parsimonious multivariate logistic model with the following predictor variables: age, renal failure, CHA2DS2-VASc score, HAS-BLED score, atrial fibrillation type, history of cerebrovascular events or coronary artery disease, vascular disease, left ventricular ejection fraction, and performance and timing of both clinical and imaging follow-up. CH: Switzerland; LAAC: left atrial appendage closure
LAAC procedure
TOE was performed before the LAAC procedure (either preprocedurally or intraprocedurally) in order to assess LAA morphology and exclude LAA thrombus12. LAAC procedures were performed according to institutional practice. In brief, all procedures were guided by means of fluoroscopy and either intracardiac echocardiography (ICE) or TOE13. In the vast majority of procedures, ICE guidance was performed under general anaesthesia. Following transvenous femoral access, transseptal puncture was performed in order to gain access to the left atrium. A device-specific sheath was advanced over a stiff 0.035” guidewire into the left atrium and directed into the proximal part of the LAA. LAA angiography was usually performed in one or more projections. A Watchman 2.5 or Watchman FLX device was implanted according to the device iteration available at the time of procedure. The procedures were comparable between the two study groups, except for device deployment, which was performed according to the related instructions for use. Watchman FLX devices were released by using the “ball technique” (placing the delivery catheter at the proximal portion of the LAA and unsheathing the distal end of device until an atraumatic distal ball was created) followed by different strategies according to the LAA morphology (device unsheathing, device advancement, or a combination of both). Watchman 2.5 devices were typically released by placing the tip of the delivery catheter distal to the landing zone and unsheathing the device. Thereafter, in both study groups, similar assessments of device stability (including a sustained tug test) and of LAA sealing were performed using echocardiography and/or angiography before device release. After the procedure, all patients routinely underwent a transthoracic echocardiography to exclude pericardial effusion before discharge14. The type and duration of the antithrombotic treatment at discharge were left to the discretion of the operator according to the individual bleeding and stroke risk151617.
Follow-up and study endpoints
TOE was performed between 1 and 6 months after the procedure in order to confirm effective LAA sealing and exclude DRT. Patients were followed by means of telephone interviews and/or outpatient visits. Source documentation of all adverse events were collected, and all events, including overall and cardiovascular death, systemic embolism, and major bleeding (Bleeding Academic Research Consortium [BARC] 3-5), were classified and adjudicated by trained cardiologists. The cerebrovascular events were reviewed by expert neurologists. The study endpoints included DRT and PDL at TOE follow-up and stroke (including both ischaemic and haemorrhagic), death, and major bleeding (BARC 3-5) at the longest available follow-up.
Both DRT and PDL were locally ascertained by trained cardiologists with extensive prior and ongoing experience in imaging LAAC devices. These cardiologists reviewed TOE images (typically in four views [0°, 45°, 90°, and 135°], with and without colour Doppler using a 50-60 cm/sec scale) and adjudicated device-related complications according to definitions established prior to event adjudication and consistent with previous publications618.
DRT was defined as a homogeneous mass with an echogenicity comparable to the myocardium on the atrial surface of the device, inconsistent with the normal healing/device incorporation process and not explained by an imaging artefact. PDL was defined as any jet adjacent to the device between the LAA and the left atrium or, alternatively, detectable by means of colour Doppler (high velocity 50-60 cm/sec).
Statistical analysis
Continuous variables are presented as mean±standard deviation. Categorical variables are reported as counts and percentages. The Student’s t-test and chi-square tests were used to assess differences between the groups. Fisher’s exact test was used when sample sizes were small (frequencies fewer than 5).
A propensity score-matched analysis was employed to minimise the baseline differences between groups. The study reporting followed previously published guidelines for reporting propensity score analysis19. The control group was defined as the Watchman 2.5 cohort after 1:1 propensity score matching (PSM) with the Watchman FLX group. The scores were estimated using a non-parsimonious multivariate logistic model with the following predictor variables: age, renal failure, CHA2DS2-VASc score, HAS-BLED score, atrial fibrillation type, history of cerebrovascular events or coronary artery disease, vascular disease, left ventricular ejection fraction, both performance and time of clinical and imaging follow-up. The greedy nearest-neighbour matching technique was used. The patients with a Watchman FLX were selected sequentially, without replacement, based on the propensity score that was closest to that of the Watchman 2.5 patients. The calliper requirement for matching was set at 0.5 (i.e., patients were matched only if the difference in the logits of the propensity score for pairs of patients from the two groups was less than or equal to 0.5 times the pooled estimate of the common standard deviation of the propensity score logit). Variable balance assessment was performed to assess the effectiveness of the matching process by estimating the standardised differences, expressed as a percentage of the pooled standard deviations between the two groups. A threshold of 0.25 for the standardised difference was used for defining balance. All differences for the matched groups were within the limits of –0.25 and 0.25, demonstrating the two groups were well balanced for the matched observations. Study endpoints were analysed by calculating the cause-specific hazard ratio (HR) without additional (i.e., other than PSM) correction. Statistical significance was considered at the 0.05 level. Statistical analyses were performed by P.G. Torlapati, an independent biostatistician working at St David’s Medical Center, Austin, TX, USA.
Results
A total of 3,424 patients were referred to the participating centres between July 2018 and January 2023 to undergo percutaneous LAAC (Figure 1). Eleven patients (0.3%) refused to be included in the local LAAC registries, and 738 patients (21.5%) received a device other than a Watchman. Of the 2,675 remaining patients, 1,257 (47.3%) received a Watchman 2.5 whereas 1,418 (52.7%) received a Watchman FLX. Of these, after performing PSM, 1,128 patients were included in each study group (Figure 1).
Baseline and procedural characteristics
Both baseline and procedural characteristics of patients who received a Watchman FLX or Watchman 2.5 before PSM are reported in Supplementary Table 1 and Supplementary Table 2. After PSM, both baseline (Table 1) and procedural (Table 2) characteristics were comparable between the two groups. The mean age was 74.7 years, and two-thirds of patients were male. The mean CHA2DS2-VASc score was 3.4, while the mean HAS-BLED score was 2.8. A previous history of cerebrovascular events or coronary artery disease were reported in 19% and 46% of patients, respectively. A history of relevant bleeding was present in one-quarter of patients (24%).
TOE was performed before and/or during LAAC in all patients, but only one-quarter of procedures were TOE-guided (24.4%): ICE was the predominant imaging technique used (75.6%). A thrombus was detected in an LAA before closure only in a small percentage of cases (0.7%). The LAAs were chicken-wing shaped in 44.3% of cases, with a mean maximum ostium diameter of 21.2 mm. However, the Watchman devices implanted in the Watchman FLX group were larger than those implanted in the Watchman 2.5 group (25.9 mm vs 24.7 mm; p<0.001). The most common discharge antithrombotic regimen was dual antiplatelet therapy (74.2%), followed by single antiplatelet therapy (10.1%) (Table 2).
Table 1. Differences in baseline patient characteristics between the two groups after propensity score matching.
| Watchman FLX (n=1,128) | Watchman 2.5 (n=1,128) | p-value | |
|---|---|---|---|
| Age, yrs | 74.5±4.9 | 74.8±4.8 | 0.149 |
| Female sex | 374 (33) | 398 (35) | 0.287 |
| BMI, kg/m² | 27.3±2.0 | 27.5±2.0 | 0.123 |
| Hypertension | 852 (75) | 883 (78) | 0.121 |
| Diabetes mellitus | 364 (32) | 394 (35) | 0.181 |
| Renal failure* | 78 (7) | 68 (6) | 0.392 |
| CHA2DS2-VASc score | 3.5±1.5 | 3.4±1.5 | 0.263 |
| HAS-BLED score | 2.8±1.2 | 2.8±1.2 | 0.173 |
| Permanent atrial fibrillation | 532 (47) | 572 (50) | 0.129 |
| History of cerebrovascular events | 204 (18) | 226 (20) | 0.238 |
| History of coronary artery disease | 508 (45) | 526 (47) | 0.447 |
| Vascular disease† | 96 (8) | 105 (9) | 0.506 |
| History of congestive heart failure | 308 (27) | 321 (28) | 0.542 |
| History of relevant bleeding‡ | 264 (23) | 281 (25) | 0.403 |
| LVEF, % | 55.5±7.1 | 55.7±7.0 | 0.399 |
| All continuous variables are presented as mean±standard deviation. Categorical variables are reported as counts and percentages. *Renal failure: <30 eGFR mL/min per 1.73m2 (using the Modification of Diet in Renal Disease formula) and/or creatinine >200 mcmol/L and/or dialysis or history of kidney transplantation. †Vascular disease was defined as the presence of any the following: history of myocardial infarction, intermittent claudication, previous surgery or percutaneous intervention on the abdominal aorta or the lower extremity vessels, abdominal or thoracic surgery, arterial or venous thrombosis. ‡History of relevant bleeding was defined as bleeding requiring medical attention and/or prompting evaluation. BMI: body mass index; eGFR: estimated glomerular filtration rate; LVEF: left ventricular ejection fraction | |||
Table 2. Differences in imaging and procedural characteristics between the two groups after propensity score matching.
| Watchman FLX (n=1,128) | Watchman 2.5 (n=1,128) | p-value | |
|---|---|---|---|
| ICE-guided | 836 (74) | 869 (77) | 0.106 |
| LAA thrombus | 10 (1) | 4 (0) | 0.108 |
| Maximum LAA ostium diameter at TOE, mm | 21.3±4.1 | 21.1±4.5 | 0.227 |
| Maximum LAA depth at TOE, mm | 25.2±7.6 | 25.4±7.1 | 0.518 |
| Chicken-wing shape | 488 (43) | 511 (45) | 0.33 |
| General anaesthesia | 963 (85) | 985 (87) | 0.177 |
| Watchman device size, mm | 25.9±7.3 | 24.7±6.2 | <0.001 |
| Device implantation attempts | 1.2±0.9 | 1.2±1.2 | 0.186 |
| Discharge antithrombotic therapy | |||
| No antithrombotic therapy | 0 | 0 | 0.241 |
| Any SAPT | 114 (10) | 125 (10) | |
| Any single anticoagulant therapy | 91 (8) | 84 (7) | |
| Any DAPT | 824 (73) | 851 (75) | |
| Any SAPT+anticoagulant therapy | 97 (9) | 115 (10) | |
| Any triple therapy | 6 (0) | 1 (0) | |
| All continuous variables are presented as mean±standard deviation. Categorical variables are reported as counts and percentages. DAPT: dual antiplatelet therapy; ICE: intracardiac echocardiography; LAA: left atrial appendage; SAPT: single antiplatelet therapy; TOE: transoesophageal echocardiography | |||
Study endpoints
Before PSM, TOE follow-up was performed less frequently (89.7% vs 92.2%; p=0.028) and at an earlier timepoint (72.63±85.10 days vs 103.52±67.54 days; p<0.001) in the Watchman FLX group compared with the Watchman 2.5 group (Supplementary Table 3). A similar pattern was observed for clinical follow-up, which was performed in 90.6% and 93.4% of patients (p=0.007) at a mean of 639 and 1,234 days (p<0.001), respectively, in the Watchman FLX and Watchman 2.5 groups (Supplementary Table 3).
After PSM, imaging and clinical follow-up were performed in all patients of both groups at a mean of 2.3 months and 1.6 years, respectively (Table 3). Both DRT (0.2% vs 3.1%; HR 0.35, 95% confidence interval [CI]: 0.21-0.38; p=0.017) and PDL (21.0% vs 30.6%; HR 0.68, 95% CI: 0.59-0.77; p=0.031) were significantly lower in the Watchman FLX group compared with the Watchman 2.5 group (Table 3). The stroke rate at the longest available follow-up was numerically lower in the Watchman FLX group compared with the Watchman 2.5 group (3.4% vs 5.1%; HR 0.56, 95% CI: 0.15-1.69; p=0.078). More specifically, the total number of strokes was 38 in the Watchman FLX group and 57 in the Watchman 2.5 group. In the Watchman FLX group, 33 strokes were ischaemic, 3 were haemorrhagic, and 2 patients experienced both types. In the Watchman 2.5 group, 48 strokes were ischaemic, 4 were haemorrhagic, and 5 patients experienced both. There was no difference between the two groups either for death (7.4% vs 9.4%; HR 0.87, 95% CI: 0.37-3.16; p=0.261) or major bleedings (HR 0.92, 95% CI: 0.76-1.37; p=0.459) (Table 3).
Table 3. Comparison of study endpoints between Watchman FLX and Watchman 2.5.
| Watchman FLX (n=1,128) | Watchman 2.5 (n=1,128) | Hazard ratio (95% confidence interval) | p-value | |
|---|---|---|---|---|
| TOE follow-up duration, days | 68.6±48.5 | 71.2±52.6 | 1.64 (0.58-4.26) | 0.613 |
| DRT | 3 (0) | 35 (3) | 0.35 (0.21-0.38) | 0.017 |
| PDL | 237 (21) | 345 (31) | 0.68 (0.59-0.77) | 0.031 |
| Clinical follow-up duration, days | 584±241 | 593±264 | 1.24 (0.95-2.53) | 0.682 |
| Stroke | 38 (3) | 57 (5) | 0.56 (0.15-1.69) | 0.078 |
| CV death, stroke, systemic embolism | 88 (8) | 113 (10) | 0.74 (0.64-1.52) | 0.814 |
| Death | 84 (7) | 106 (9) | 0.87 (0.37-3.16) | 0.261 |
| CV death | 64 (6) | 81 (7) | 0.72 (0.32-2.70) | 0.260 |
| Systemic embolism | 7 (1) | 11 (1) | 0.95 (0.87-1.96) | 0.861 |
| Major bleeding (BARC 3 or 5) | 94 (8) | 115 (10) | 0.92 (0.76-1.37) | 0.459 |
| All continuous variables are presented as mean±standard deviation. Categorical variables are reported as counts and percentages. BARC: Bleeding Academic Research Consortium; CV: cardiovascular; DRT: device-related thrombus; PDL: peridevice leak; TOE: transoesophageal echocardiography | ||||
Discussion
The Watchman FLX device, after obtaining CE mark approval in 2019 and FDA approval in 2020, has progressively replaced the older-generation Watchman 2.5 in clinical practice. The new device iteration was introduced to the market with the aim of improving procedural outcomes and reducing device-related complications such as DRT and PDL, and consequently the risk of ischaemic stroke. While procedural and long-term clinical outcomes appear to have improved with the newer version, it remains unclear whether the two device iterations are associated with different risks of device-related complications.
In this large dual-centre prospectively collected cohort of consecutive successful percutaneous LAAC procedures with either a Watchman FLX or a Watchman 2.5, after PSM we observed the following (Central illustration):
1) The implantation of a Watchman FLX was associated with lower rates of DRT and PDL at early TOE follow-up.
2) The stroke rate at approximately 1.5 years after the index procedure was numerically lower in the Watchman FLX group compared with the Watchman 2.5 group.
DRT represents a major LAAC device-related complication. According to previous large-scale studies, it occurs in approximately 2-5% of cases and is associated with a 2-5-fold increased risk of ischaemic events at follow-up20. We observed a significantly higher frequency of DRT in the Watchman 2.5 group compared with the Watchman FLX group. To date, there are three studies comparing these two devices in terms of DRT. Paitazoglou et al compared a multicentre cohort of 164 consecutive patients receiving a Watchman FLX device with a matched Watchman 2.5 cohort from the EWOLUTION registry (n=1,025)21. At TOE follow-up, the authors observed a numerically, albeit non-significantly, lower rate of DRT in the Watchman FLX group compared with the Watchman 2.5 group (2.4% vs 3.7%; p=0.5)21. Accordingly, the WATCH-DUAL study, including a dual cohort of 144 consecutive LAAC procedures implanting either a Watchman 2.5 or FLX device, showed a significantly lower rate of DRT at imaging follow-up (0% vs 6%; p=0.045) including TOE and/or computed tomography9. However, because of the small size of the above studies and the lack of results adjustment of the latter, their observations could be considered as hypothesis-generating only. On the other hand, Price et al recently reported an analysis of the United States National Cardiovascular Data Registry (NCDR) LAAO Registry comparing the outcomes of LAAC procedures completed in the United States with the implantation of Watchman FLX between August 2020 and June 2021 and an identical number of patients receiving the Watchman 2.5 at the same sites directly preceding the first Watchman FLX case at each site11. The study cohort consisted of 27,141 patients receiving each device and the investigators used both multivariate analysis and PSM (2:1) in order to compare the two study groups. Unlike what we observed in our study, the investigators reported a similar rate of DRT between the Watchman FLX and Watchman 2.5 groups (0.4% vs 0.5%; p=0.27)11. One or more of the following four points might explain the inconsistency with our results. First, the TOE timing in the NCDR study was 45 days compared with 70 days in our trial, which may have increased the DRT detection rate, since DRTs are reportedly more frequent beyond this point in time. Second, the antithrombotic therapy at discharge was significantly different between the two study groups in the NCDR, which may have influenced the DRT results. Third, in our study, the definition of DRT was standardised, and TOE images were reviewed by trained cardiologists with extensive previous and ongoing imaging work on LAAC, which had the potential advantage of reducing the risk of event underreporting. Indeed, our DRT rates were consistent with those reported in the largest multicentre studies to date, while those in the NCDR registry were much lower2223. Fourth, the NCDR did not report data about potential confounders such as imaging guidance, LAA size, LAA morphology, presence of LAA thrombus, or number of device implantation attempts (i.e., all variables that were well balanced between our study groups after PSM), making it impossible to assess the potential impact of the LAAC procedure iteration (irrespective of the device) on their study results. From a mechanistic point of view, it still remains unclear if the lower DRT risk associated with the Watchman FLX is related to either the more proximal device implantation allowed by its design (i.e., better device conformability and shorter length of the device lobe) or to the less thrombogenic flat atrial device surface (i.e., inverted screw end and less metal exposed)9, or, presumably, both factors together. Since the device implantation depth was not assessed in our study, this question will remain unanswered. However, since proximal implantation is also an operator-dependent factor, our observations, along with findings from previous studies24, suggest that LAAC operators might be encouraged to target a proximal implantation of the Watchman FLX device, as it may reduce the risk of DRT.
PDL is the most frequent LAAC device-related complication and is associated with an increased thromboembolic risk. Paitazoglou et al reported that Watchman FLX implantation was associated with a significantly higher LAA sealing rate as compared with Watchman 2.5 at 3 months after LAAC (90% vs 79.4%; p=0.039)21. Similar results were recently shown by Price et al11. Our observations are in line with these results. However, unlike the above studies, both morphology and size of the LAAs (i.e., potential risk factors for residual PDL) were well balanced between the study groups in our study. Furthermore, unlike the ALSTER-FLX Registry, in our study, the LAAC operators and the imaging experts who assessed the PDL at follow-up did not differ between the two study groups21. Finally, we evaluated the impact of procedural iteration and the operator learning curve on the incidence of PDL and DRT, but we found no significant associations (Supplementary Appendix 1). The improved LAA sealing capability of the Watchman FLX compared with its previous iteration may be attributable to several factors. First, the additional row of 18 J-shaped barbs and the increased number of strut frames in the Watchman FLX likely enhances the conformability of the device and the device-to-LAA wall contact. Second, the device oversizing and the consequent greater device compression (suggested by the use of larger device sizes in the FLX group despite similar ostial LAA dimensions between groups) allowed by the FLX device design may reduce the risk of side gap development during the months after the procedure25. Third, the longer fabric outer membrane of the Watchman FLX may reduce the risk of a so-called “mixed leak”, where the passage of blood might occur partially at the sides of the device and partially through the device after the membrane ends6.
The lower rate of device-related complications in the Watchman FLX group compared with the Watchman 2.5 group was accompanied by a trend towards a lower stroke rate in the Watchman FLX group. It is unclear if the observed trend is the result of a real risk difference between the devices or a random finding. However, the recent observation by Price et al, showing an approximately 18% reduction in stroke risk associated with the use of the Watchman FLX compared with the previous iteration (adjusted HR 0.816, 95% CI: 0.683-0.975; p=0.0253), along with the differing rates of device-related complications between our study groups, suggests that a higher statistical power might have allowed us to detect a significant difference.
Our study did not focus on procedural safety since the comparison of the two Watchman devices in terms of procedure-related complications was properly addressed by another substudy of the NCDR LAAO Registry that showed, in a consecutive cohort of approximately 50,000 LAAC procedures, a significantly lower rate of in-hospital major adverse events for the Watchman FLX compared with the Watchman 2.5 (1.35% vs 2.40%; adjusted odds ratio [OR] 0.57, 95% CI: 0.50-0.65; p<0.0001), driven largely by fewer pericardial effusions requiring intervention (0.42% vs 1.23%; adjusted OR 0.34, 95% CI: 0.28-0.42; p<0.001)10.
The current observations, in line with the previous largest prospective studies conducted with Watchman 2.523 and FLX26, in addition to the evidence accumulated so far regarding the superiority of the Watchman FLX in terms of procedural and long-term safety1011, consistently support the notion of a continued improvement of the Watchman device.
The reduced thrombogenic risk, the improved sealing capability, and the enhanced safety profile of the new Watchman FLX compared to its predecessor may support a de-escalation of the current antithrombotic therapy recommended in the device instructions for use following device implantation, which was mainly based on studies involving the previous iteration, the Watchman 2.5. This is especially important if we consider the number of major bleedings during follow-up that might be prevented in such a high bleeding risk population as that submitted to LAAC.
Finally, the device-related complications observed in the Watchman FLX group are not negligible, and there is still room for improvement27. The device will soon be replaced by the Watchman FLX PRO, which features a membrane coated with antiplatelet drugs and is expected to further reduce the risk of DRT in the future28.
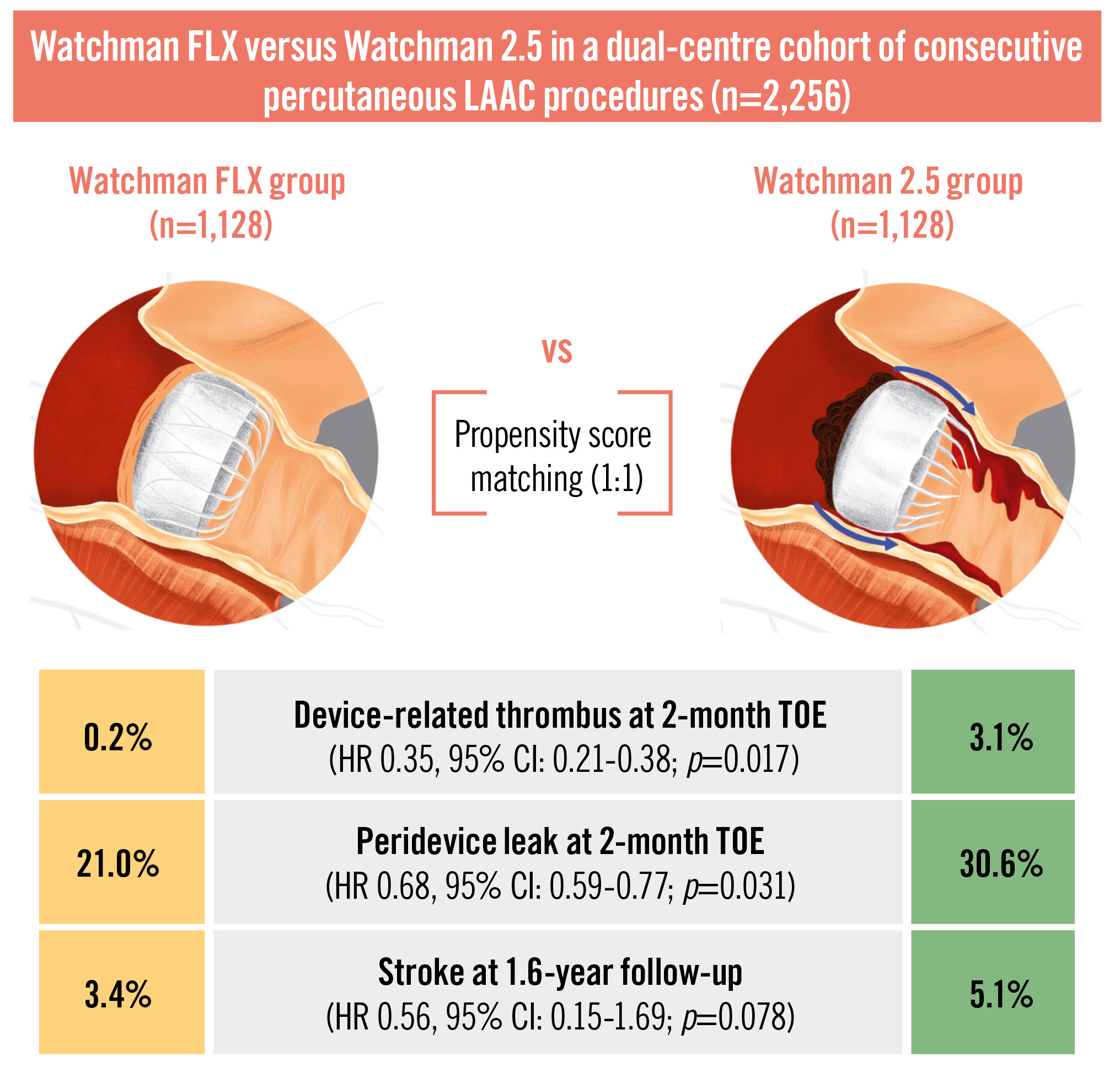
Central illustration. The main results of the WATCH-MATCH analysis. CI: confidence interval; HR: hazard ratio; LAAC: left atrial appendage closure; TOE: transoesophageal echocardiography
Limitations
Our study has several limitations. The observational and retrospective design of the study may have introduced bias and unmeasured confounders, for which the propensity score matching analysis may have not accounted. The Watchman FLX replaced the Watchman 2.5 at the participating centres in 2019-2020, so we cannot exclude that the operator learning curve and the LAAC procedure iteration might have affected the comparison between the two groups. However, the vast majority of procedures were performed by operators with high experience with the Watchman 2.5 (i.e., >35 procedures prior to enrolling patients in the study), and no significant association was observed between the occurrence of device-related complications and operator learning curve surrogates (Supplementary Appendix 1). Furthermore, the procedures were performed with an identical technique between the two study groups, with the only exceptions being device sizing and device deployment, and all procedural and LAA anatomical characteristics were well balanced between the two groups after PSM. Moreover, if we consider only the two years (2019-2020) during which both device iterations were available (a relatively short period in which the impact of LAAC evolution on device-related complications might be negligible), we observed a significantly lower rate of PDL in 2019 (27.7% vs 5.3%; p=0.03) and a lower rate of DRT in 2020 (3.0% vs 0.5%; p=0.046) in the Watchman FLX group compared with the Watchman 2.5 group, respectively (Supplementary Figure 1).
Neither imaging nor clinical follow-up was centrally assessed. Yet, the definitions used by each participating centre were agreed upon by both teams and standardised before the start of endpoint adjudication, and they are in line with those recommended by the latest consensus documents29.
Conclusions
In a large dual-centre cohort of consecutive, successful LAAC procedures using two iterations of the Watchman device, the Watchman FLX was associated with significantly lower rates of both DRT and PDL compared with the Watchman 2.5. Our observational data provide scientific support for the continued improvement of Watchman device iterations.
Impact on daily practice
A growing body of evidence indicates that the Watchman FLX is associated with a lower risk of device-related complications compared with its predecessor, the Watchman 2.5. Its reduced thrombogenic potential and enhanced sealing properties may justify de-escalating the antithrombotic regimen currently recommended in the device’s instructions for use, which are primarily based on studies involving the previous iteration.
Conflict of interest statement
R. Galea is a proctor and consultant for Abbott; has received speaker honoraria from Boston Scientific; and he reports research grants to the institution from the Swiss Heart Foundation. L. Roten has received research grants from Medtronic, the Swiss National Science Foundation, the Swiss Heart Foundation, the Immanuel and Ilse Straub Foundation, and the Sitem Insel Support Fund, all for work outside the submitted study; and he has received speaker fees/honoraria from Biosense Webster, Boston Scientific, Abbott, and Medtronic. L. Di Biase is a consultant for Biosense Webster, Boston Scientific, Stereotaxis, and St. Jude Medical; and has received speaker honoraria from Medtronic, AtriCure, EpiEP, and Biotronik. A. Natale is a consultant for Biosense Webster, Abbott, Medtronic, Biotronik, Boston Scientific, iRhythm, Field Medical, and Pulse Biosciences. L. Räber reports research grants to institution by Abbott, Boston Scientific, Biotronik, Boston Scientific, Infraredx, HeartFlow, Sanofi, Swiss National Science Foundation, Swiss Heart Foundation, and Regeneron; and he reports speaker/consultation fees by Abbott, Amgen, CSL Behring, Canon, Novo Nordisk, Medtronic, Occlutech, and Sanofi. The other authors have no conflicts of interest relevant to the contents of this paper to declare.
Supplementary data
To read the full content of this article, please download the PDF.

