Cory:
Unlock Your AI Assistant Now!
Abstract
Secondary mitral regurgitation (SMR) is frequent among patients with heart failure (HF) with reduced ejection fraction (HFrEF), and it is strongly associated with increased mortality, frequent hospitalisations, and poor quality of life. The mechanisms underlying SMR are multifactorial. While guideline-directed medical therapy and cardiac resynchronisation therapy remain the cornerstone of HFrEF management, many patients with significant SMR continue to experience significant symptoms and adverse outcomes. Managing SMR within the context of HF necessitates a multifaceted approach. Transcatheter edge-to-edge repair (TEER) has emerged as a transformative intervention, demonstrating improvements in survival, functional capacity, and HF-related hospitalisations in clinical trials and real-world registries in selected patients. This review provides a comprehensive overview of the evidence supporting TEER, focusing on procedural and follow-up outcomes, and its role in reshaping the therapeutic approach for HF patients with SMR. Additionally, we highlight the critical role of patient selection and identify predictors of poor outcomes as key determinants of TEER success.
Mitral regurgitation (MR) and heart failure (HF) represent a dynamic and complex interaction of valvular and myocardial dysfunction, with each condition amplifying the adverse effects of the other1. HF, which affects over 64 million people globally, remains a leading cause of hospitalisations and mortality worldwide23. Concurrently, MR is the most prevalent valvular heart disorder, with moderate to severe MR present in more than 50% of patients with acute HF456. The coexistence of MR in patients with HF imposes an additional haemodynamic burden, intensifying symptoms and increasing hospitalisation rates, and it is strongly associated with poor outcomes7. The presence of MR is closely associated with disease severity and adverse outcomes in HF8.
MR is categorised into primary (degenerative or organic) and secondary (functional) forms. Primary MR (PMR) results from intrinsic structural abnormalities in the mitral valve (MV) apparatus, including the leaflets, chordae tendineae, papillary muscles, or mitral annulus. In contrast, traditional secondary MR (SMR) results from geometric distortion of the MV apparatus due to left ventricular (LV) remodelling and dysfunction, frequently secondary to ischaemic or non-ischaemic cardiomyopathies9. This form of MR is particularly challenging, as it both reflects the severity of the underlying myocardial disease and exacerbates HF progression through chronic volume overload and elevated left atrial (LA) pressure1011. However, atrial remodelling can contribute to SMR, and the new concept of atrial SMR is characterised by significant LA dilation, impaired LA function, and mitral annular dilation, often with preserved LV function1213. SMR poses unique clinical challenges due to its bidirectional impact on HF progression. It independently predicts higher mortality in HF patients, with severity closely correlating with worse clinical outcomes14. Managing SMR within the context of HF necessitates a multifaceted approach, as it serves not only as a marker of advanced myocardial disease but also as a possible therapeutic target to mitigate disease progression and improve survival.
Surgical intervention remains the cornerstone for managing PMR, with durable MV repair or replacement often yielding favourable long-term outcomes15. However, in SMR, the role of surgery remains less well defined because no conclusive evidence from a well-designed randomised trial has demonstrated a survival benefit in patients with isolated SMR1617. Furthermore, a substantial proportion of SMR patients are deemed unsuitable for surgery due to advanced age, significant LV dysfunction, and multiple comorbidities18. As a result, the management of SMR has traditionally relied on conservative strategies, including guideline-directed medical therapy (GDMT), cardiac resynchronisation therapy (CRT), and, in advanced cases, the use of ventricular assist devices or heart transplantation19.
In recent years, transcatheter therapies of the mitral valve, particularly mitral transcatheter edge-to-edge repair (TEER), have emerged as a transformative therapeutic option, particularly for patients at high surgical risk. Landmark trials in patients with SMR and HF have provided pivotal insights into the efficacy and safety of TEER, catalysing a paradigm shift in the management of SMR202122. However, discrepancies in their findings underscore the importance of meticulous patient selection and the nuanced interplay between MR severity and LV dysfunction. This state-of-the-art review delves into the evolving role of TEER, with a focus on SMR, highlighting its growing evidence base and its potential to reshape outcomes for this high-risk population.
MV anatomy, pathophysiology and assessment of secondary MR
The MV apparatus comprises the annulus, surrounding myocardium, leaflets, chordae tendineae, and papillary muscles, functioning as an integrated and dynamic unit essential for maintaining optimal cardiac function. The MV apparatus and the LV are closely interdependent, with changes in one structure invariably affecting the other23.
Structural or functional abnormalities in the MV impact LV performance, while morphological or functional changes in the LV have reciprocal consequences on the MV competence. Importantly, the MV is not a passive structure merely responding to cardiac forces; its annulus exhibits active sphincteric properties that not only contribute to ventricular contractility but are also significantly influenced by it. Given this intricate interplay and structural complexity, a comprehensive understanding of the MV anatomy and its surrounding structures is critical for optimal patient selection and the successful execution of TEER in SMR.
SMR arises in the setting of a structurally normal MV and results from abnormalities in the MV apparatus that are driven by LV or LA remodelling. SMR is broadly categorised into ventricular SMR (V-SMR) and atrial SMR (A-SMR), with V-SMR accounting for the majority of the cases24 (Figure 1). V-SMR is typically associated with ischaemic or non-ischaemic cardiomyopathy25. In V-SMR, LV dysfunction or dilation disrupts the balance between reduced closing forces because of impaired LV contractility and annular contraction and increased tethering forces caused by LV enlargement, papillary muscle displacement, or dyskinesia. Ischaemic V-SMR often results from myocardial infarction, where regional remodelling of the infarcted segment tethers the mitral leaflets, causing incomplete coaptation and regurgitation. In contrast, non-ischaemic V-SMR results from diffuse LV dilation and global remodelling, leading to symmetrical leaflet tethering and central MR jets.
A-SMR is an increasingly recognised subset of SMR, characterised by isolated mitral annular dilation and LA enlargement, often occurring in the context of preserved LV geometry and function and normal MV leaflet morphology26. These factors lead to impaired leaflet coaptation and tethering, causing significant MR and HF. A-SMR is commonly associated with atrial fibrillation (AF) and heart failure with preserved ejection fraction (HFpEF), both of which share overlapping risk factors and contribute to adverse LA remodelling27. A-SMR reflects a complex bidirectional relationship between LA myopathy and HFpEF, where elevated filling pressures drive LA dilation and dysfunction, exacerbating MR28 (Figure 1). The increasing prevalence of AF and HFpEF is expected to drive a significant rise in cases of A-SMR.
A-SMR poses significant challenges in interpreting existing registry data and clinical trials. Most historical studies did not specifically differentiate between A-SMR and V-SMR, making it difficult to draw definitive conclusions about TEER outcomes in pure A-SMR populations29. However, recent studies provide encouraging evidence supporting TEER as a promising treatment option for A-SMR303132. A-SMR is particularly challenging in patients with HF with mildly reduced ejection fraction (HFmrEF; left ventricular ejection fraction [LVEF] 40-49%), where both atrial and ventricular mechanisms may contribute to the development of MR33. The overlap becomes even more complex in patients with AF, as it can be difficult to determine whether the atrial remodelling is the primary driver of MR or a consequence of underlying ventricular dysfunction34. Furthermore, two distinct subtypes of A-SMR have been identified: one characterised by isolated mitral annular dilation (ASMR-IAD) and another by atriogenic hamstringing (ASMR-AH), with ASMR-AH showing worse procedural and clinical outcomes following TEER than ASMR-IAD35. This heterogeneity in A-SMR phenotypes further complicates the interpretation of existing evidence and highlights the need for more precise patient classification in future studies.
The lack of standardised criteria for distinguishing A-SMR from V-SMR, particularly in HFmrEF patients, represents a significant gap in current knowledge and may impact patient selection and outcomes following TEER. This underscores the importance of developing more refined diagnostic criteria and conducting dedicated studies specifically focused on A-SMR populations. These knowledge gaps likely account for the absence of A-SMR in current ACC/AHA guidelines and previous European Society of Cardiology (ESC) clinical guidelines3637. However, the newly updated 2025 ESC guidelines suggest that TEER may be considered for symptomatic A-SMR (Class IIb)38.
Overall, both forms of SMR contribute to chronic volume overload, neurohormonal activation, and elevated atrial and pulmonary pressures, contributing to adverse LV remodelling and thereby perpetuating a vicious cycle of HF exacerbation and reducing survival24. While A-SMR patients often maintain preserved LV function, the majority with V-SMR experience HF with reduced ejection fraction (HFrEF), representing a high-risk subgroup among those with LV dysfunction39. Understanding the natural progression of SMR is essential for determining the ideal timing for intervention. The cyclical interaction of impaired LV function, progressive LV dilation, and SMR drives LA enlargement, predisposing patients to AF and secondary pulmonary hypertension. Sustained volume overload and haemodynamic stress further aggravate the condition, culminating in secondary tricuspid regurgitation, right ventricular (RV) dysfunction, and ultimately biventricular failure if left untreated40. Furthermore, the MV itself undergoes significant structural changes in response to the mechanical stress of SMR, rather than remaining a passive bystander. The valve actively adapts through leaflet growth and remodelling, attempting to maintain adequate coaptation despite the altered geometric relationships41. However, this adaptive response can become maladaptive over time, accelerating the progression of regurgitation and thereby creating a vicious cycle.
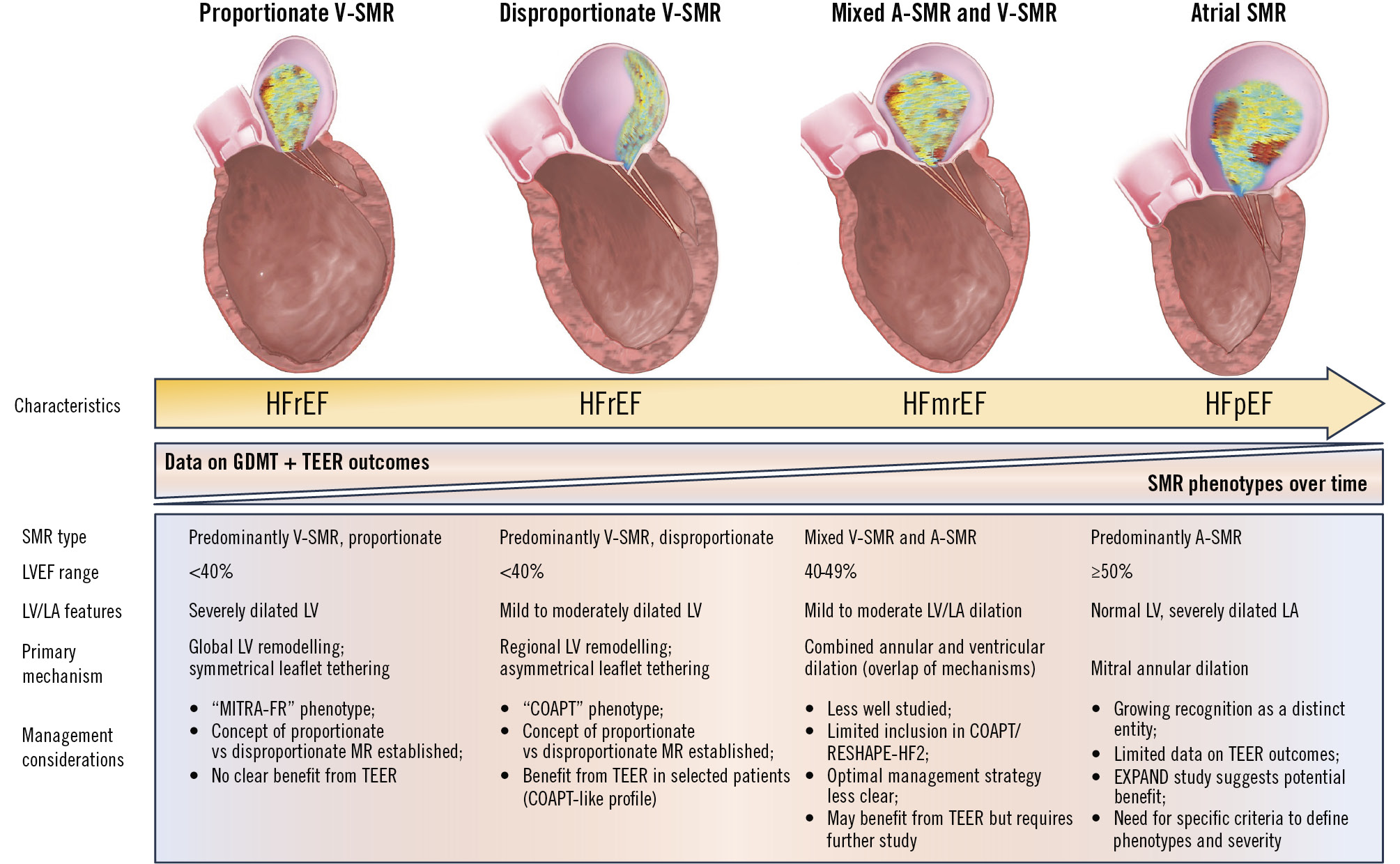
Figure 1. SMR phenotypes across the spectrum of heart failure. This figure illustrates the shifting landscape of SMR phenotypes across HF subtypes based on EF: proportionate V-SMR in HFrEF, disproportionate V-SMR in HFrEF, mixed A-SMR and V-SMR mechanisms in HFmrEF, and predominantly atrial SMR in HFpEF. The timeline highlights the evolving data on GDMT and TEER outcomes, demonstrating how improved medical therapy has reduced the prevalence of V-SMR while increasing the recognition of A-SMR. Management considerations and primary mechanisms are detailed for each phenotype, emphasising the importance of tailored interventions. A-SMR: atrial secondary mitral regurgitation; COAPT: Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation; EF: ejection fraction; EXPAND: Contemporary, Prospective Study Evaluating Real-world Experience of Performance and Safety for the Next Generation of MitraClip Devices; GDMT: guideline-directed medical therapy; HF: heart failure; HFmrEF: heart failure with mildly reduced ejection fraction; HFpEF: heart failure with preserved ejection fraction; HFrEF: heart failure with reduced ejection fraction; LA: left atrium; LV: left ventricle; LVEF: left ventricular ejection fraction; MITRA-FR: Percutaneous Repair with the MitraClip Device for Severe Functional/Secondary Mitral Regurgitation; RESHAPE-HF2: A Randomised Study of the MitraClip Device in Heart Failure Patients with Clinically Significant Functional Mitral Regurgitation; SMR: secondary mitral regurgitation; TEER: transcatheter edge-to-edge repair; V-SMR: ventricular secondary mitral regurgitation
Assessment of SMR
Echocardiography is the fundamental imaging modality for diagnosing and evaluating SMR, playing a pivotal role in assessing its severity and suitability for TEER. Transthoracic echocardiography is often the first-line modality due to its ease of use and reproducibility. However, transoesophageal echocardiography is often required for more detailed assessment, particularly in cases with poor acoustic windows, eccentric MR jets, mixed aetiologies (e.g., leaflet thickening or mitral annular calcification), or the need for quantitative measurements. Current guidelines define severe MR by an effective regurgitant orifice area (EROA) ≥0.40 cm2, regurgitant volume ≥60 mL/beat, or regurgitant fraction ≥50%, though lesser thresholds may still carry prognostic significance3638. When feasible, the proximal isovelocity surface area (PISA) method is a highly valuable tool for quantifying the severity of MR, as it can be applied to both central and eccentric jets. In the context of SMR, the thresholds for significant MR associated with adverse outcomes and requiring intervention may be lower. An EROA ≥30 mm² and/or a regurgitant volume ≥45 mL/beat are often considered clinically significant, especially in the presence of an elliptical regurgitant orifice or low-flow conditions. These values align with moderate to severe MR, but the regurgitant fraction generally remains ≥50%, consistent with severe MR classification42. Additionally, a PISA radius of ≥1 cm is also recognised as a criterion for assessing the severity of MR43. However, these assessments are not without limitations. For example, the dynamic nature of SMR can cause variability in regurgitation severity based on haemodynamic loading conditions (such as with sedation during transoesophageal echocardiography). Furthermore, methods like the PISA calculation are prone to error in SMR due to non-hemispherical or elliptical mitral valve orifices, conical PISA shapes, and eccentric or multiple MR jets, which can result in underestimation of regurgitant severity44. On the other hand, single-frame EROA measurements during mid-systole may overestimate MR severity in biphasic patterns with early or late peaks45.
Multimodality imaging is crucial in overcoming these limitations and addressing these challenges42. Three-dimensional (3D) echocardiography offers superior accuracy in measuring the vena contracta and provides a more comprehensive evaluation of the MV anatomy, particularly for complex or eccentric regurgitant jets46. Within the context of TEER, 3D colour Doppler echocardiography facilitates spatial localisation of the optimal repair site, thereby enhancing procedural planning46. Additionally, cardiac magnetic resonance may be considered for its ability to provide precise measurements of regurgitant volume, particularly in cases with complex jet dynamics47. A meticulous preprocedural echocardiographic assessment remains indispensable for evaluating the anatomical feasibility of TEER, including parameters such as leaflet tethering, coaptation distance, and LV geometry.
TEER devices for secondary MR
MV surgery in SMR requires careful patient selection and timing. While surgical intervention has a clear role in patients undergoing concomitant cardiac surgery, its use as an isolated procedure should be individualised because of the significant associated risk36373848. Surgical intervention has not consistently demonstrated an ability to alter the natural course of primary conditions, such as dilated cardiomyopathy, nor has it improved survival outcomes, particularly in cases involving irreversible ventricular remodelling or pulmonary hypertension, which are associated with a high risk of adverse outcomes, including a reduction in LVEF4950. These limitations have driven the development of transcatheter therapies, offering less invasive alternatives with promising clinical outcomes. Over the past decade, TEER has rapidly advanced, addressing the unmet clinical need for improved management of MR in the high-risk HF population (Figure 2). The primary objective of TEER is to improve leaflet coaptation and reduce the regurgitation orifice area, by mimicking the surgical Alfieri edge-to-edge stitch technique. Currently, TEER is the most widely adopted approach for treating HF patients with SMR.
Among the two commercially available and approved TEER devices, the MitraClip (Abbott) is approved for the treatment of both SMR and PMR, while PASCAL (Edwards Lifesciences) is primarily indicated for PMR. Clinical evidence predominantly stems from investigations of the MitraClip device, although these findings may be theoretically extended to the broader TEER approach. Hence, this review focuses on the MitraClip and PASCAL devices. Over 200,000 patients worldwide have undergone TEER5152, and its efficacy and safety in patients with HF and SMR have been evaluated in three major randomised trials: Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation (COAPT), Percutaneous Repair with the MitraClip Device for Severe Functional/Secondary Mitral Regurgitation (MITRA-FR), and the recent Randomised Investigation of the MitraClip Device in Heart Failure: Second Trial in Patients with Clinically Significant Functional Mitral Regurgitation (RESHAPE-HF2)202122. Both the ACC/AHA 2020 and the ESC 2021 guidelines for valvular heart disease recommend TEER as a Class IIa indication for patients with severe, symptomatic SMR who meet the COAPT Trial criteria. For patients not meeting these criteria, the 2021 ESC guidelines suggested TEER may still be considered a Class IIb recommendation to alleviate symptoms and enhance quality of life37. In the new 2025 ESC guidelines, the recommendation for symptomatic SMR has been strengthened and TEER has been upgraded from Class IIa to Class I (Level of Evidence A).38(Figure 2).
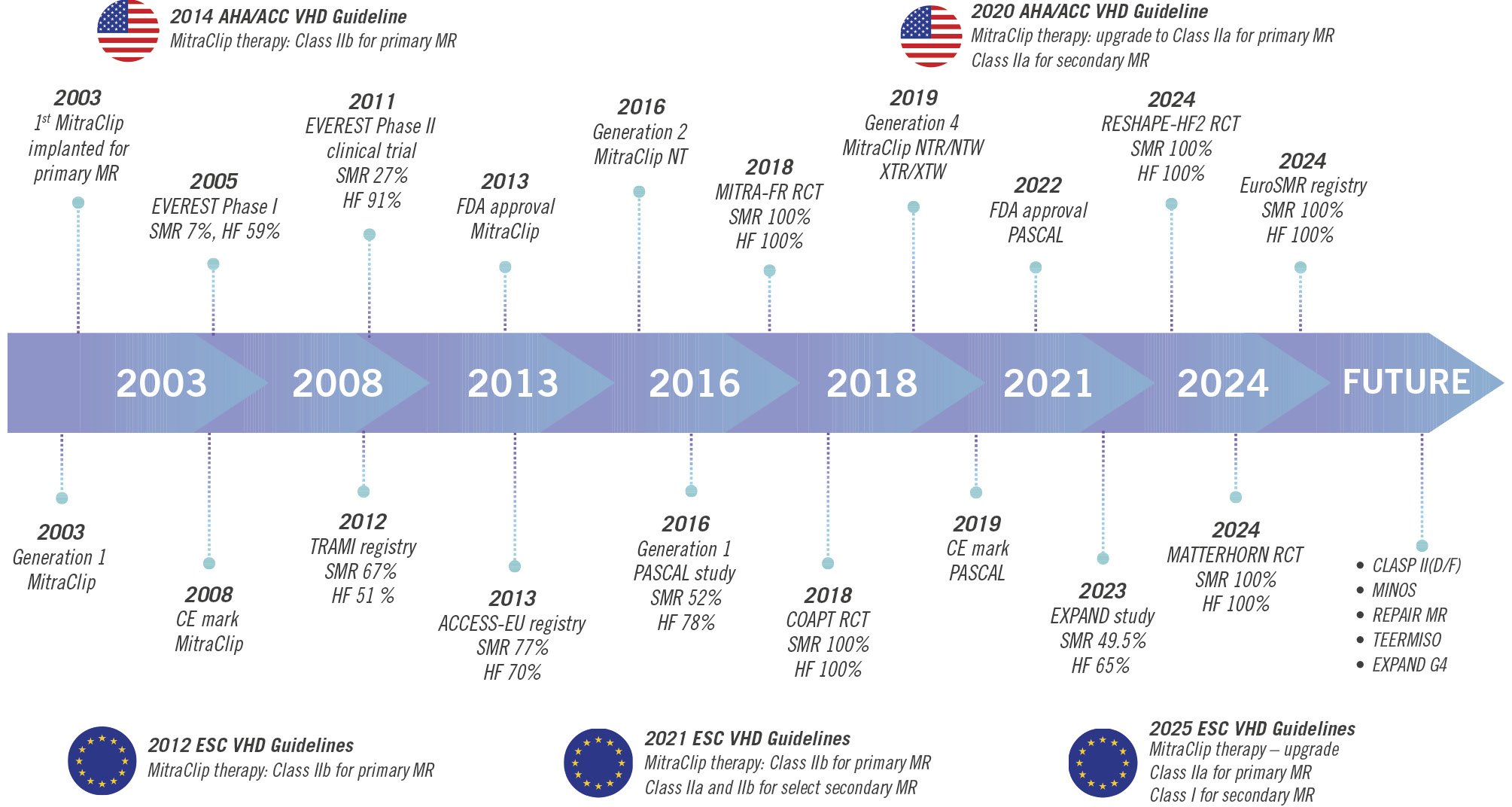
Figure 2. Timeline of key milestones in TEER and guideline evolution for mitral regurgitation treatment. This timeline illustrates the significant advancements in TEER for MR and its integration into clinical practice guidelines. ACCESS-EU: MitraClip Therapy Economic and Clinical Outcomes Study Europe; MINOS: Transcatheter Mitral Valve Repair for Inotrope Dependent Cardiogenic Shock; CE: European Conformity; COAPT: Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation; ESC: European Society of Cardiology; EVEREST: Endovascular Valve Edge-to-Edge Repair Study; EXPAND G4: Clinical Evaluation of the MitraClip Generation 4 System in a Contemporary Real-World Population; FDA: U.S. Food and Drug Administration; HF: heart failure; MATTERHORN: Multicenter, Randomized, Controlled Study to Assess Mitral Valve Reconstruction for Advanced Insufficiency of Functional or Ischemic Origin; MITRA-FR: Percutaneous Repair with the MitraClip Device for Severe Functional/Secondary Mitral Regurgitation; MR: mitral regurgitation; NYHA: New York Heart Association; RCT: randomised clinical trial; REPAIR-MR: Percutaneous MitraClip Device or Surgical Mitral Valve Repair in Patients with Primary Mitral Regurgitation Who Are Candidates for Surgery; RESHAPE-HF2: A Randomised Study of the MitraClip Device in Heart Failure Patients with Clinically Significant Functional Mitral Regurgitation; SMR: secondary mitral regurgitation; TEER: transcatheter edge-to-edge repair; TEERMISO: Transcatheter Versus Standard Surgical Mitral Valve Operation for Secondary Mitral Regurgitation; TRAMI: transcatheter mitral valve interventions; VHD: valvular heart disease
MitraClip
The MitraClip is the most extensively used TEER device for MR. It approximates the anterior and posterior mitral leaflets to create a double orifice and reduce regurgitant volume. While the initial Endovascular Valve Edge-to-Edge Repair Study (EVEREST) Phase I and II trial provided early safety data, it included a highly selected population and is not reflective of current practice5354. The evolution to the fourth-generation MitraClip G4 system has introduced several meaningful improvements aimed at enhancing precision and flexibility in more complex anatomies. These include four distinct device configurations – NT, XT, and the wider NTW and XTW versions – that allow customisation based on leaflet length and coaptation gap. Importantly, the G4 enables independent leaflet grasping, providing better control when targeting asymmetrical leaflet motion or uneven tethering. The addition of real-time left atrial pressure monitoring during the procedure allows for intraoperative assessment of haemodynamic impact and aids decision-making (Figure 3)5556.
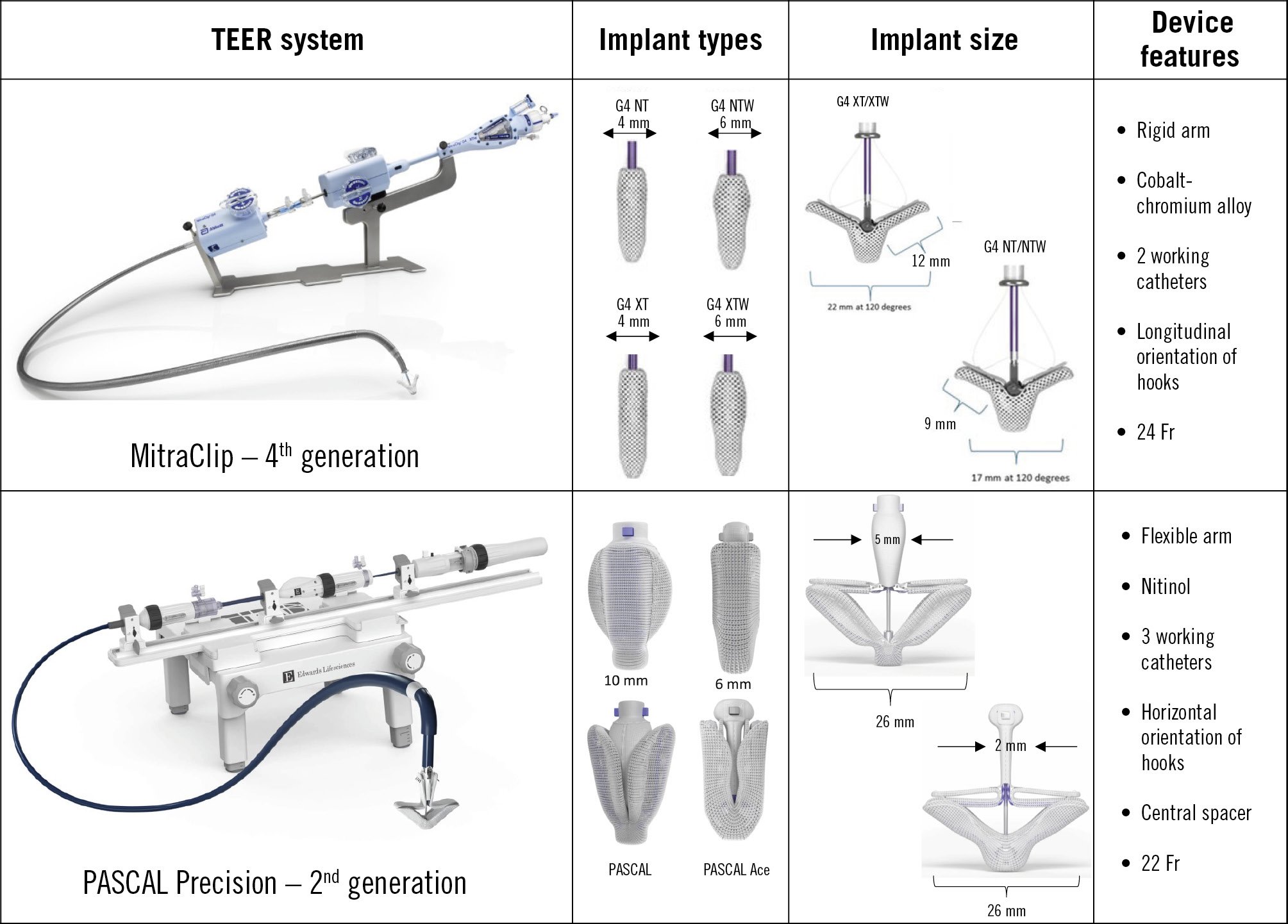
Figure 3. Comparison of TEER systems: MitraClip 4th generation and PASCAL Precision 2nd generation. This figure provides a comparative overview of two TEER systems and their key features. TEER: transcatheter edge-to-edge repair; XTW: extra tall wide
PASCAL
The PASCAL system is the second most widely used TEER device. It uses broad, contoured paddles with an integrated central spacer and clasps to approximate the mitral leaflets and reduce regurgitation. The spacer helps fill the regurgitant orifice and distributes stress across a larger leaflet area, potentially reducing leaflet trauma (Figure 3). After demonstrating promising outcomes in early pilot studies5758, further safety and efficacy data were reported in the CLASP study (n=109), in which 67% of patients had SMR and over half were in New York Heart Association (NYHA) III/IV. At 1 year, the study demonstrated 92% survival and 88% freedom from heart failure hospitalisation (HFH), with 82% of patients having MR reduced to ≤1+ and all to ≤2+. Patient quality of life, measured by the 12-item Kansas City Cardiomyopathy Questionnaire (KCCQ-12) score, improved by an average of 14 points59.
The PASCAL Ace is a lower-profile variant of the original device. It incorporates a narrower spacer (2 mm vs 5 mm) and slimmer paddles (Figure 3). The reduced spacer size improves manoeuvrability and enables use in combination with additional devices without inducing mitral stenosis or device entanglement60. In the CLASP IID randomised trial, PASCAL demonstrated non-inferiority to MitraClip in patients with PMR, with similar procedural success, MR reduction, and 1-year outcomes6162.
Randomised trials
Over the past decade, three landmark randomised trials, COAPT, MITRA-FR, and RESHAPE-HF2, have shaped the evidence base for TEER in the treatment of SMR in patients with symptomatic HFrEF. While these trials collectively highlight the potential of TEER, they also reveal significant heterogeneity in patient selection, echocardiographic characteristics, and clinical outcomes, underscoring the complexity of managing this heterogeneous condition. Current ACC/AHA guideline recommendations for SMR primarily reference two of these trials, COAPT and MITRA-FR, both published in 2018. However, the recently published RESHAPE-HF2 trial adds critical new evidence on this topic, which has already influenced the updated 2025 ESC guidelines38, further refining the role of TEER in managing SMR in patients with HF. Table 1 summarises trial characteristics and outcomes.
The COAPT Trial enrolled patients with moderate-to-severe (3+) or severe (4+) SMR, with a mean EROA of 0.40 cm2 and mild to moderate LV dilation, with a mean left ventricular end-diastolic volume (LVEDV) of 193 mL. In this cohort, TEER combined with GDMT demonstrated substantial reductions in HFHs (35.8% vs 67.9%; hazard ratio [HR] 0.53, 95% confidence interval [CI]: 0.40-0.70; p<0.001) and all-cause mortality (29.1% vs 46.1%; HR 0.62, 95% CI: 0.46-0.82; p<0.001) at 2 years compared to GDMT alone, alongside notable improvements in quality of life and exercise capacity, with these benefits sustained over a 5-year follow-up period2063. The findings from COAPT underscore the efficacy of TEER in patients with severe MR that is “disproportionate” to the degree of ventricular dilation, wherein the pathology is primarily localised to the MV rather than being secondary to LV dysfunction.
In contrast, the MITRA-FR trial enrolled patients with a broader profile, including individuals with less severe MR, where the degree of regurgitation was “proportionate” to the extent of advanced ventricular remodelling. Thus, the typical patient in the COAPT Trial exhibited “disproportionately severe” MR, while the majority of patients in the MITRA-FR trial presented with “proportionately severe” or less severe MR64. The inclusion of patients with proportionate MR, where LV dilation is the predominant driver of regurgitation, likely diluted the efficacy signal of TEER in MITRA-FR. As a result, the trial showed no significant benefit in reducing all-cause mortality or HFHs at 1 year (54.6% vs 51.3%; odds ratio [OR] 1.16, 95% CI: 0.73-1.84; p=0.53), and this remained consistent at 2 years2165. Interestingly, the MITRA-FR post hoc analysis66 challenged the widely held “proportionate-disproportionate” hypothesis, which posits that the degree of benefit from TEER depends on the relationship between MR severity and LV remodelling. While COAPT demonstrated significant benefit in patients with disproportionate MR, the MITRA-FR post hoc analysis failed to show improved outcomes in any subgroup, even among those with large EROAs and smaller LV volumes (disproportionate MR phenotype), characteristics that closely align with the COAPT population. This discrepancy may be explained by several factors including lower procedural success rates (88% vs 95% in COAPT), less stringent patient selection, and differences in medical therapy optimisation between the trials. Additionally, unmeasured factors such as differences in RV function, the extent of LV fibrosis, or contractile reserve may have played a significant role in influencing the contrasting outcomes observed in the two studies. These findings highlight the complexity of interpreting trial data and suggest that factors beyond the proportionate-disproportionate framework may critically influence TEER outcomes.
The RESHAPE-HF2 trial22 offered an intermediate perspective, enrolling patients with potentially less severe SMR and characteristics more similar to those in COAPT, albeit with slightly larger LV volumes. The mean EROA was 0.25 cm², mean regurgitant volume was 37 mL, and the mean LVEDV of 205 mL was smaller than in MITRA-FR. Despite these differences, TEER demonstrated significant benefits across all three co-primary endpoints in the RESHAPE-HF2 trial. Over 2 years, cardiovascular death and all HFHs were reduced by 36% (p=0.002), while all HFHs were reduced by 41% (p=0.002). Additionally, the quality of life, assessed using the KCCQ overall summary score, improved by 10.9 points at 1 year (p<0.001). Significant improvements in NYHA Functional Class were also observed at 30 days, and 6, 12, and 24 months (p<0.0001). However, mortality outcomes did not reach statistical significance22. A prior HFH within the 12 months preceding study enrolment was associated with worse overall outcomes but also appeared to correlate with a more pronounced response to TEER. Perhaps most notably, no apparent relationship was observed between the baseline severity of MR, as assessed by EROA tertiles, and the 2-year relative reduction in the rate of HFHs achieved with TEER. Functional improvements, including gains in the 6-minute walk distance and KCCQ scores, were consistent with those seen in COAPT. Nevertheless, the lack of a survival benefit raises questions regarding the durability of TEER benefits in this patient cohort, particularly in those with advanced LV remodelling. Patients undergoing TEER (vs GDMT alone) experienced significantly fewer days lost due to death or HFH during the follow-up period (1,067 days vs 1,776 days; p<0.0001). Ponikowski et al67 described their study population as having moderate to severe MR; however, the reported EROA values (mean EROA of 0.25 cm2, with only 14% having an EROA>0.40 cm2 and 23% having an EROA <0.20 cm2)68 suggest that a significant proportion of participants, if not the majority, may have had only moderate MR. Notably, 66% of participants experienced an HFH within the year prior to enrolment. Overall, the trial’s findings suggest that while TEER can effectively enhance the quality of life and decrease the frequency of HFHs more than medical therapy alone, it did not demonstrate a survival benefit at midterm follow-up in patients with mild to moderate LV remodelling and less severe MR (Figure 4).
The contrasting outcomes observed in COAPT, MITRA-FR, and RESHAPE-HF2 highlight the importance of understanding the distinction between proportionate and disproportionate MR in the management of SMR64. Disproportionate MR, characterised by regurgitation severity that exceeds what is expected for the degree of LV dilation, has been proposed as a useful framework to identify patients more likely to benefit from TEER, particularly in the COAPT-like population. However, patients in RESHAPE-HF2 exhibited less severe MR and intermediate LV volumes between those of COAPT and MITRA-FR, and therefore may not strictly meet the criteria for disproportionate MR. Although the framework of proportionate and disproportionate MR offers a compelling theoretical rationale for divergent trial results, it has never been shown to yield any meaningful clinical difference when selecting patients for TEER. As highlighted by Adamo et al, its limited utility is likely due to the dynamic nature of SMR, with patients classified as proportionate in one echocardiogram and disproportionate in another, depending on loading conditions69. More recent evidence suggests that excessive LV remodelling, particularly with LVEDV >200-220 mL or LV diameters >65-70 mm, may attenuate the prognostic benefit of TEER, highlighting the importance of LV geometry as an independent predictor of outcomes7071. Figure 5 shows the relationship between EROA and LVEDV across key trials and registries evaluating SMR. Interestingly, Kubo et al demonstrated that poor LV function and significant remodelling were associated with suboptimal MR reduction, and residual MR ≥2+ was linked to worse clinical outcomes72.
Unlike COAPT, MITRA-FR, and RESHAPE-HF2, which compared TEER to GDMT in symptomatic HF patients with SMR, no prior randomised trial had compared TEER with MV surgery in patients with HFrEF and SMR. This cohort, characterised by significantly higher perioperative risk than the EVEREST II population, had previously been understudied until the recently published Multicentre, Randomized, Controlled Study to Assess Mitral Valve Reconstruction for Advanced Insufficiency of Functional or Ischemic Origin (MATTERHORN) trial. It is the first randomised trial to compare surgical MV intervention with TEER in patients with isolated SMR73. A total of 208 patients were enrolled across 16 German centres, with a mean age of 70.5 years, a mean LVEF of 43.0%, and 83% experiencing HFH within the previous year. As depicted in Figure 5, the EROA and LVEDV observed in the MATTERHORN trial were notably smaller compared to those reported in previous trials. At 1 year, the trial demonstrated non-inferiority of TEER compared to surgery for a composite primary endpoint of death, HFH, reintervention, LV assist device implantation, or stroke (16.7% vs 22.5% in the TEER and surgical groups, respectively; p<0.001 for non-inferiority). Both treatment arms showed significant improvements in functional status, with fewer patients in NYHA Class III/IV at 1 year (23.3% for TEER vs 18.2% for surgery). While surgical repair achieved marginally greater reductions in MR grade and recurrence rates, TEER effectively maintained MR ≤2+ in 96% of patients, with a superior procedural safety profile.
Importantly, the trial reinforces TEER’s role as a viable alternative to surgery, especially in light of the absence of conclusive randomised trial evidence supporting survival benefits with surgery in isolated SMR. This divergence is reflected in the guidelines: the 2021 ESC guidelines reserved TEER for patients deemed inoperable36, whereas the 2020 ACC/AHA guidelines offered a Class IIa recommendation for TEER as first-line therapy in symptomatic patients (NYHA Class II-IV) with LVEF 20-50% despite GDMT37. However, the new 2025 ESC guidelines now closely align with the ACC/AHA stance, upgrading TEER to a Class I, Level of Evidence A recommendation for carefully selected patients with ventricular SMR persisting after optimal medical therapy, recognising TEER as the recommended option in this population38. The neutral results of MATTERHORN and TEER’s favourable safety profile further support this harmonised approach in clinical practice, particularly for high-risk populations While the MATTERHORN trial provides valuable insights, its findings require cautious interpretation when applied to HF phenotypes, particularly HFmrEF and HFpEF73. The trial’s inclusion of patients with LVEF ≥20% without an upper limit uniquely allowed for the representation of HFmrEF and HFpEF populations, with a mean LVEF of 43% aligning within the HFmrEF range. This broad inclusion highlighted the prevalence of mixed SMR mechanisms (A-SMR and V-SMR) but complicates the process of drawing specific conclusions for HFmrEF and HFpEF groups. Furthermore, the high proportion of MV replacement (28%) in the surgical group points to more complex valve pathologies, further blurring distinctions between functional subtypes. Clinically, the intersection of A-SMR and V-SMR in HFmrEF and HFpEF complicates patient selection, the optimal timing of intervention, and the choice between TEER and surgery. The traditional proportionate/disproportionate framework developed for V-SMR in HFrEF does not fully capture the nuances of A-SMR, which is driven by atrial remodelling, LA volume, and mitral annular dilation. While TEER benefits appear consistent across LVEF ranges, uncertainty remains at higher LVEF thresholds (>50%), reflecting gaps in our understanding of its role in HFmrEF and HFpEF. Moving forward, individual patient-level meta-analyses and the integration of advanced imaging techniques will be essential to further refine patient selection criteria and optimise clinical outcomes.
Table 1. Key characteristics and outcomes of TEER versus medical therapy trials in secondary mitral regurgitation.
| COAPT (201820) | MITRA-FR (201821) | RESHAPE-HF2 (202422) | |
|---|---|---|---|
| Sample size | 614 (302 TEER, 312 control) | 304 (152 TEER, 152 control) | 505 (250 TEER, 255 control) |
| Inclusion criteria | (1) LVEF 20-50%, (2) NYHA Class II-IV, (3) EROA ≥0.3 cm², (4) HF hospitalisation within 12 months | (1) LVEF 15-40%, (2) NYHA Class II-IV, (3) EROA ≥0.2 cm², (4) HF hospitalisation or BNP/NT-proBNP | (1) LVEF 20-50%, (2) NYHA Class II-IV, (3) EROA ≥0.2 cm², (4) HF hospitalisation or BNP/NT-proBNP |
| Age, years | 71±11 | 70±10 | 70±10 |
| Female | 221 (36.0) | 77 (25.3) | 99 (19.6) |
| Ischaemic aetiology | 60.7 | 59.2 | 65 |
| HFH within the previous 1 year | 351 (57.2) | 304 (100) | 333 (65.8) |
| LVEF, % | 31±9 | 33±6 | 31±8 |
| LVEDV, mL | 193 | 250 | 205 |
| EROA, cm² | 0.41±0.15 | 0.31±0.10 | 0.25 |
| GDMT | Maximally tolerated, independent committee to monitor | Community management per European guidelines | Optimally managed (investigator-assessed) |
| 2-year mortality | |||
| TEER | 29.1# | 34.9 | 22.3# |
| Control | 46.1 | 34.2 | 29.6 |
| 2-year HFH | |||
| TEER | 35.8* | 55.9 | 26.9^ |
| Control | 67.9 | 61.8 | 46.6 |
| Symptoms change | ¯ symptoms | « symptoms | ¯ symptoms |
| Data are provided as mean, mean±SD, %, or n (%). #Event rates are Kaplan-Meier estimates; *annualised rate (in % per year) within 2-year follow-up; ^events per 100 patient-years. : increase; ¯: reduction; « : no change. BNP: B-type natriuretic peptide; COAPT: Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation; EROA: effective regurgitant orifice area; GDMT: guideline-directed medical therapy; HF: heart failure; HFH: heart failure hospitalisation; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; MITRA-FR: Percutaneous Repair with the MitraClip Device for Severe Functional/Secondary Mitral Regurgitation; MR: mitral regurgitation; NT-proBNP: N-terminal pro-B-type natriuretic peptide; NYHA: New York Heart Association; RESHAPE-HF2: A Randomized Study of the MitraClip Device in Heart Failure Patients with Clinically Significant Functional Mitral Regurgitation; SD: standard deviation; TEER: transcatheter edge-to-edge repair | |||
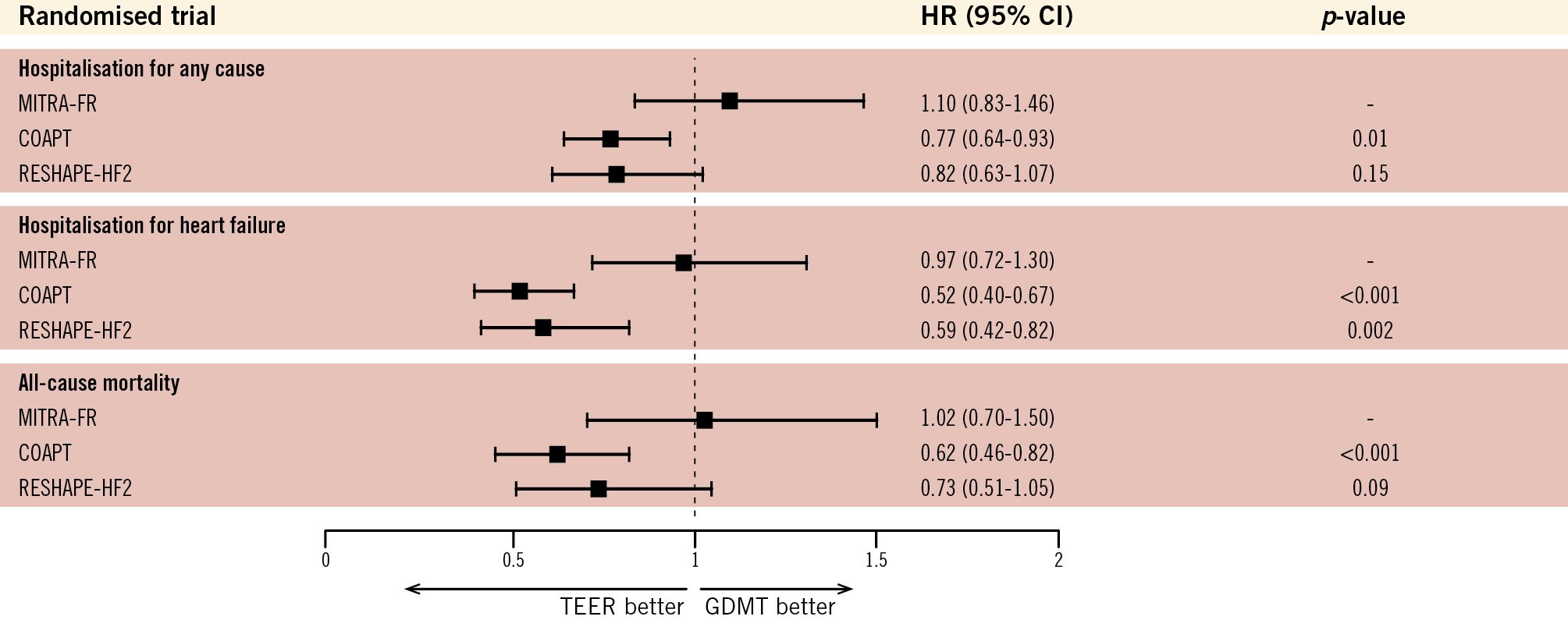
Figure 4. Comparative outcomes of TEER and GDMT across randomised trials in heart failure with secondary mitral regurgitation. The figure shows HRs with 95% CIs for hospitalisation (all-cause and HF-specific) and all-cause mortality. COAPT demonstrated significant reductions in HF hospitalisations and all-cause mortality with TEER, while MITRA-FR and RESHAPE-HF2 showed mixed results, reflecting differences in patient selection, trial design, and severity of SMR. The dashed line (HR=1) represents equivalence between TEER and GDMT, with HRs below 1 favouring TEER. CI: confidence interval; COAPT: Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation; GDMT: guideline-directed medical therapy; HF: heart failure; HR: hazard ratio; MITRA-FR: Mitral Clip Versus Medical Therapy in Patients With Severe Secondary Mitral Regurgitation; RESHAPE-HF2: A Randomised Study of the MitraClip Device in Heart Failure Patients With Clinically Significant Functional Mitral Regurgitation; TEER: transcatheter edge-to-edge repair
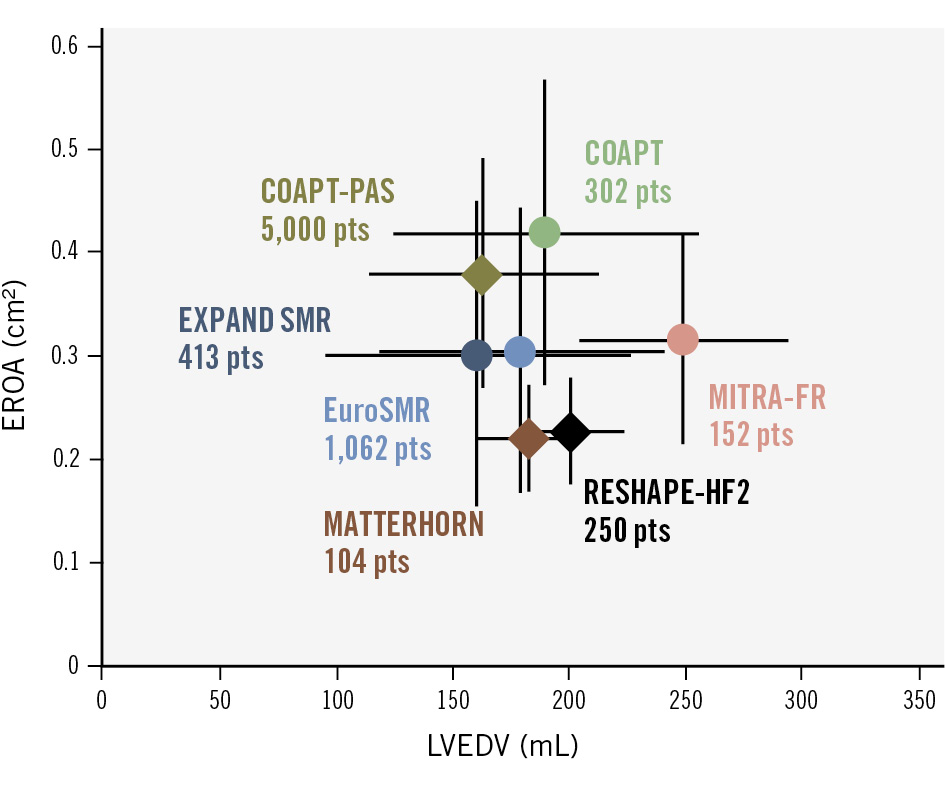
Figure 5. Distribution of SMR proportionality across major TEER trials and registries. Adapted from Stolz et al125. This figure demonstrates the relationship between EROA and LVEDV across key trials and registries evaluating SMR. Patients (pts) from the COAPT Trial predominantly exhibited disproportionate SMR, while the MITRA-FR trial enrolled patients with proportionate SMR. RESHAPE-HF2 and MATTERHORN trials were more similar in terms of their patients with proportionate SMR, though patients had less severe mitral regurgitation and slightly smaller LV volumes. Real-world registries, including EuroSMR and EXPAND SMR, indicate a predominance of disproportionate SMR among participants. Each data point represents the mean±standard deviation (circles) or median with interquartile range (diamonds). COAPT: Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation; COAPT PAS: COAPT Post-Approval Study; EROA: effective regurgitant orifice area; EuroSMR: European Registry of Transcatheter Repair for Secondary Mitral Regurgitation; LVEDV: left ventricular end-diastolic volume; MATTERHORN: Multicenter, Randomized, Controlled Study to Assess Mitral Valve Reconstruction for Advanced Insufficiency of Functional or Ischemic Origin; MITRA-FR: Multicentre Study of Percutaneous Mitral Valve Repair MitraClip Device in Patients With Severe Secondary Mitral Regurgitation; RESHAPE-HF2: A Randomised Study of the MitraClip Device in Heart Failure Patients with Clinically Significant Functional Mitral Regurgitation; SMR: secondary mitral regurgitation; TEER: transcatheter edge-to-edge repair
Evidence beyond randomised trials
Real-world data on the effectiveness of TEER in SMR has been provided by multicentre registries, which consistently report significant symptomatic improvements and reductions in HFH following the commercial approval of TEER systems. Building on the foundation of the EVEREST II trial54, multiple registries, with a predominant focus on SMR, have demonstrated the feasibility and efficacy of the MitraClip system in this patient population. These studies consistently report substantial symptomatic improvements and reduced HFHs74. Figure 2 illustrates the timeline of key milestones, while Table 2 summarises the major registries for TEER in SMR7475767778798081828384. The MitraSwiss registry included 560 patients with SMR (46.2%) and 652 with PMR (53.8%). Compared with PMR patients, SMR patients were younger, had more comorbidities, and had worse LV function. Procedural success rates were high, at over 91% in both groups, with similar reductions in MR grade and improvements in the NYHA Functional Class. However, the cumulative probability of death at 5 years was higher in the SMR cohort (54%) compared to the PMR cohort (45%; p=0.009). Interestingly, the aetiology of MR was not an independent predictor of poor outcomes. Instead, independent predictors of mortality included older age, anaemia, impaired renal function, and reduced LVEF, reflecting the more systemic nature of HF in SMR patients81.
The German transcatheter mitral valve interventions (TRAMI) registry included 1,064 MitraClip-treated patients (71% SMR, 69% LVEF <50%). At 3 months, 66% achieved NYHA Class I-II, though 12% were rehospitalised for HF and one-third remained symptomatic83. Despite improved function and quality of life, 4-year mortality was high (53.1%), reflecting advanced disease and comorbidities typical of real-world SMR populations84. Similarly, data from a US registry revealed comparable outcomes, with approximately one-quarter of patients experiencing mortality and nearly one-fifth requiring rehospitalisation for HF. These adverse outcomes were strongly associated with advanced age, impaired LV function, SMR, and residual MR following the procedure85.
The Contemporary, Prospective, Multicentre Study Evaluating Real-world Experience of Performance and Safety for the Next Generation of MitraClip Devices (EXPAND) study included patients with more complex mitral anatomies and utilised the third-generation MitraClip NTR/XTR system. This device offered enhanced leaflet grasping and procedural flexibility, expanding the applicability of TEER to patients previously deemed unsuitable because of anatomical challenges. The study enrolled 1,041 patients across 57 international centres, with 50% presenting with SMR, of whom 65% had a history of HFH. Procedural success was remarkably high (98.9%), and durable MR reduction was achieved, with 93% of SMR patients attaining MR severity ≤1+ at 1 year. Annualised HFH rates significantly reduced from 108% pre-TEER to 38% post-TEER in SMR patients (p<0.001), demonstrating the potential of TEER to mitigate the HF burden. Despite this improvement, HFHs remained higher in SMR patients (38%) compared to PMR patients (21%)86. This discrepancy may be attributed to the underlying pathophysiology of SMR, where LV dysfunction and adverse remodelling remain the primary drivers of HF progression, even after successful MR reduction. PMR patients, in contrast to those who typically have a structurally normal LV, derive greater haemodynamic benefits from MR correction alone. Among SMR patients in the EXPAND study, the 1-year combined all-cause mortality and HFH rate was 18.9%, showcasing the effectiveness of the third-generation MitraClip. In the ongoing EXPAND G4 analysis − a prospective, multicentre, single-arm observational study evaluating the MitraClip G4 system87 − 1-year mortality rates were even lower at 14.2%, aligning with real-world outcomes from the COAPT Post-Approval Study (COAPT-PAS)88. These improved outcomes can be attributed to multiple factors, including pharmacological advancements in HF management, particularly for patients with reduced LVEF, enhanced patient selection strategies, and greater MR reduction achieved with the newer TEER device. Additionally, operator experience and improved imaging technologies have contributed to procedural precision and long-term success, reducing mortality and HF burden in both PMR and SMR patients.
Additionally, the largest observational study of MitraClip in real-world practice, the COAPT-PAS, evaluated 5,000 SMR patients treated across 406 US centres within the Transcatheter Valve Therapy (TVT) registry. Compared to the randomised COAPT Trial, patients in COAPT-PAS had more comorbidities, more advanced HF, greater functional limitations, and less optimised GDMT. Despite these challenges, clinical outcomes were comparable or better, with a 1-year mortality rate of 22.2% (vs 19.1% in COAPT; p=0.20) and a significantly lower rate of HFH (18.9% vs 24.9%; p=0.02). Patients experienced durable MR reduction, substantial quality-of-life improvements, and similar adverse event rates88.
Regarding the durability of outcomes after the TEER procedure, the study by Adamo et al89 involved a smaller cohort (n=304) and focused exclusively on mortality following TEER, lacking follow-up visits to evaluate clinical or echocardiographic outcomes. In contrast, Kalbacher et al84 examined long-term outcomes in a larger cohort of 722 patients undergoing TEER, reporting sustained improvements in NYHA Functional Class and self-rated health status at a median follow-up of 2.8 years. However, comprehensive, large-scale datasets validating the long-term outcomes of COAPT in real-world settings remained limited until the results of the European Registry of Transcatheter Repair for Secondary Mitral Regurgitation (EuroSMR) registry were reported. This registry provided critical long-term data on a high-risk population of 1,628 HF patients with SMR across 14 European centres79, offering valuable insights into the durability of TEER in advanced HF populations. Overall, 86.6% of patients had NYHA Functional Class III/IV, indicating progressive HF symptoms, and a mean LVEF of 36%, with 19% of patients having HFpEF. The durability of MR reduction was notable, with 85.5% of patients achieving MR grade ≤2+ at 5 years post-TEER. However, the 5-year survival rate was 35.0%, notably lower than the 42.7% observed in the COAPT Trial, reflecting the inclusion of a more advanced HF population in real-world practice. The high 5-year mortality rate (65.0%) underscores the persistent risks associated with this high-risk cohort and highlights the necessity for additional therapeutic strategies alongside TEER to address the underlying HF and associated comorbidities.
The updated 5-year results of the COAPT Trial further confirmed the long-term benefits of TEER. Patients in the intervention group showed significantly reduced annual rates of HFH (33.1% vs 57.2%; HR 0.53, 95% CI: 0.40-0.68) and all-cause mortality (57.3% vs 67.2%; HR 0.72, 95% CI: 0.58-0.89). Device-related safety events were minimal (1.4%), all within the first 30 days post-procedure63.
Table 2. Key characteristics and outcomes of patients undergoing MitraClip implantation in different registries.
| Source | Inclusion period | Patients, no. | Mean age, yrs | Logistic EuroSCORE | Mean baseline LVEF, % | Secondary MR aetiology, % | History of HF, % | NYHA Class III-IV, % | Procedural success, % | 1-year all-cause mortality, % | 1-year NYHA I-II, % | 1-year MR ≤2+, % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EVEREST II High Risk Study (Whitlow et al)75 | 2005-2008 | 78 | 76.7±9.8 | 14.2±8.2# | 54.4±13.7 | 59 | 100 | 89.8 | 96 | 24.4 | 74.1 | 77.8 |
| ACCESS-EU (Maisano et al)76 | 2009-2011 | 567 | 73.7±9.6 | 23±18.3 | 52.7 (≤40% LVEF) | 77.1 | 70.1 | 84.9 | 91.2 | 17* | 71.4 | 78.9 |
| TCVT Sentinel Pilot Registry (Nickenig et al)77 | 2011-2012 | 628 | 74.2±9.7 | 20.4±16.7 | 42.6±15.9 | 72 | - | 85.5 | 95.4 | 15.3 | 74.2 | 94 |
| TRAMI (Puls et al)74 | 2010-2013 | 749 | 76 | 20 | 32.8 (<30% LVEF) | 71.3 | 51 | 89 | 97 | 17.3 | 66 | - |
| MARS (Tay et al)78 | 2011-2014 | 163 | 71.5±11.8 | 17.7±14.7 | 36±12 | 54 | - | 78.4 | 95.5 | 4.5^ | 78.2^ | 83^ |
| EuroSMR (Stocker et al)79 | 2008-2020 | 1,628 | 73.8±9.7 | 6.9±8.1f | 36.0±13.1 | 100 | 100 | 60.1 | 94 | 27.1† | 34.9† | 85.5† |
| GRASP (Scandura et al)80 | 2008-2013 | 180 | 71.6±9.8 | 7.6±6.4 | 48.35 (<30% LVEF) | 81.7 | - | 81.1 | 97.8 | 12.2 | 71 | 85.9 |
| MitraSwiss (Sürder et al)81 | 2011-2018 | 560 | 76 [69-81.5] | 8.4 [4.1-20.7]# | 36 [28-50] | 100 | 37.2 | 72.8 | 91.6 | 3.3^ | 77.7^ | 76.8^ |
| Godino C et al82 | 2008-2016 | 314 | 69±16.5 | 18.5±18 | 30.8±10 | 100 | 100 | 78 | 80 | 10†† | 66†† | 86†† |
| STS/ACC TVT (Goel et al)88 | 2019-2020 | 5 | 73.1±11.3 | 8.9±7.5ff | 37±14.7 | 100 | 64.4 | 85.6 | 93.8 | 22.2 | 77.3 | 90.7 |
| Data are provided as mean, mean±SD, median [IQR], n, or n (%). #Mean or median STS-PROM (%); fEuroSCORE II; ffSTS mitral valve replacement score; *mortality rate for SMR patients; ^at 30 days; ††at 2 years; †at 5 years. ACCESS-EU: MitraClip Therapy Economic and Clinical Outcomes Study Europe; EuroSCORE: European System for Cardiac Operative Risk Evaluation; EuroSMR: European Registry of Transcatheter Repair for Secondary Mitral Regurgitation; GRASP: Getting Reduction of Mitral Insufficiency by Percutaneous Clip Implantation; HF: heart failure; IQR: interquartile range; LVEF: left ventricular ejection fraction; MARS: MitraClip Asia-Pacific Registry; MitraSwiss: Swiss Registry of MitraClip Procedures; MR: mitral regurgitation; NYHA: New York Heart Association; SD: standard deviation; SMR: secondary mitral regurgitation; STS: Society of Thoracic Surgeons; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality; TCVT: transcatheter valve treatment; TRAMI: transcatheter mitral valve interventions; TVT: Transcatheter Valve Therapy | ||||||||||||
Role of GDMT/CRT, patient selection, and predictors of adverse outcome
Optimisation of GDMT and assessment for CRT represent the foundational first steps in the management of patients with HFrEF, including those with SMR, and the current guidelines recommend full GDMT uptitration prior to consideration of TEER3638. Similarly, patients who meet the criteria for CRT should undergo device implantation before evaluation for mitral repair, as CRT can itself reduce MR severity through ventricular resynchronisation and reverse remodelling90. Long-term administration of GDMT has been shown to reverse LV remodelling, potentially improving MV leaflet coaptation and reducing the severity of MR91. Indeed, nearly 60% of SMR patients with reduced LV function demonstrated significant improvement in SMR following GDMT, emphasising its critical role as the first-line therapy before considering advanced interventions92. The advent of sacubitril/valsartan and sodium-glucose cotransporter-2 inhibitors (SGLT2i) has significantly transformed SMR management in HFrEF by inducing reverse cardiac remodelling and reducing SMR severity93. The Pharmacological Reduction of Functional, Ischemic Mitral Regurgitation (PRIME) randomised study demonstrated a 30% reduction in EROA with sacubitril/valsartan compared to valsartan alone94. Although both PRIME and COAPT investigated therapies for SMR, they enrolled distinct patient populations and assessed different endpoints. PRIME included lower-risk patients with milder MR and a 1-year mortality of <2%, while COAPT studied higher-risk patients with advanced HF and a 1-year mortality nearing 23% in the GDMT arm. Additionally, PRIME focused on echocardiographic outcomes, whereas COAPT evaluated clinical endpoints. These fundamental differences preclude direct comparisons between the trials. The Prospective Study of Biomarkers, Symptom Improvement, and Ventricular Remodeling During Sacubitril/Valsartan Therapy for Heart Failure (PROVE-HF) study showed that 50% of patients with moderate to severe MR improved to mild MR or less within 12 months, with considerable reverse cardiac remodelling95. Similarly, dapagliflozin has shown benefits in MR reduction and ventricular remodelling in patients with SMR on GDMT96. It is important to consider that all the major TEER trials were conducted before the widespread implementation of these medications. Thus, optimising modern GDMT and reassessing patients before considering TEER remains essential for achieving the best outcomes. While GDMT has demonstrated efficacy in reversing LV remodelling and reducing SMR in select patients, this improvement often occurs early in the disease course and may not be sustained. Notably, in the COAPT Trial, approximately 30% of patients experienced recurrent significant MR after initial improvement with medical therapy alone. These findings highlight the limitations of relying solely on pharmacological treatment and support the timely consideration of TEER in appropriately selected patients.
Moderate to severe SMR affects up to one-third of HF patients eligible for CRT, with 30-40% experiencing persistent or worsening SMR post-CRT implantation97. Patients with severe SMR that remains unresolved post-CRT face higher morbidity and mortality compared with those with reduced or resolved SMR98. As demonstrated by Auricchio et al, TEER has proven effective in CRT non-responders, establishing MitraClip as a safe and feasible option for SMR reduction. TEER significantly improved NYHA Functional Class, MR grade, and reverse LV remodelling99. Similarly, a substudy from COAPT, analysing outcomes based on prior CRT use, showed significant improvements in NYHA Functional Class, MR grade, and LV remodelling in CRT-treated patients. Interestingly, patients without prior CRT also exhibited favourable outcomes, including a 12.2% improvement in LVEF to >35%100. These findings suggest that patients with moderate-severe or severe SMR should undergo careful reassessment with repeat echocardiography within 2 to 3 months following CRT. If symptoms persist with grade 3+ or 4+ SMR, TEER should be considered to improve prognosis and quality of life. The recent Transcatheter Mitral Valve Repair in non-Responders to Cardiac Resynchronization Therapy (MITRA-CRT) randomised trial focused on CRT non-responders with persistent SMR. Although the sample size was small (31 patients), it showed that TEER significantly reduced the combined endpoint of cardiac death, heart transplantation, and HFH (13% vs 67% in the control group; p=0.003) and achieved durable MR reduction (85% with MR ≤2) at 1 year compared with GDMT. Functional outcomes, including improved NYHA Class and 6-minute walk distance, further validated TEER’s efficacy in this challenging population101.
Importantly, TEER may serve as a therapeutic enabler by facilitating the uptitration of GDMT in patients with HFrEF who were previously intolerant to optimal medical therapy because of haemodynamic compromise102. Evidence from the EuroSMR registry demonstrated that GDMT uptitration occurred in more than one-third of patients following successful TEER103. This included the initiation or dose escalation of beta blockers, angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), angiotensin receptor-neprilysin inhibitors (ARNIs), and/or mineralocorticoid receptor antagonists (MRAs) after the procedure and was independently associated with lower rates of all-cause mortality and HFH. Notably, patients who achieved GDMT intensification after TEER exhibited improved long-term survival and reduced HF-related morbidity compared with those who did not. The ability of TEER to reduce MR and stabilise haemodynamics creates a clinical window for intensifying GDMT, underscoring the synergistic value of mechanical and pharmacological interventions in HF care.
This benefit of TEER and GDMT appears particularly relevant in patients with RV dysfunction, a well-established predictor of adverse outcomes in secondary mitral regurgitation. Recent findings from the EuroSMR registry indicate that the prognostic benefit of GDMT uptitration following TEER extends to patients with RV impairment, with significant improvements in long-term survival observed in this subgroup104. Mechanistically, TEER may alleviate pulmonary congestion and reduce RV afterload by mitigating mitral regurgitation, indirectly contributing to stabilisation of RV function. In this high-risk population, careful preprocedural assessment and post-intervention optimisation are critical. Echocardiographic indicators such as tricuspid regurgitation severity, pulmonary artery pressure, and RV strain may assist in guiding patient selection and tailoring follow-up strategies. These findings reinforce the importance of integrated care strategies that target both left-sided and right-sided dysfunction in patients undergoing TEER.
Despite this, significant heterogeneity in TEER outcomes persists, driven largely by variations in patient characteristics and disease progression. As a result, patient selection has emerged as a key determinant of TEER success105106. To date, COAPT is the only randomised trial demonstrating a survival benefit of TEER compared with conservative management. Adamo et al showed that patients with a COAPT-like profile undergoing TEER achieved superior long-term outcomes compared with non-COAPT-like patients107. Among 452 patients, 42.3% met COAPT-like criteria. Over a median follow-up of 3.6 years, COAPT-like patients had significantly lower all-cause mortality (50.8% vs 73.6%; p<0.001) and HFH (56.4% vs 77.1%; p<0.001). Multivariable analysis confirmed a COAPT-like profile as an independent predictor of better survival (HR 0.59, 95% CI: 0.47-0.74; p<0.001)107. These findings underscore the importance of selecting suitable patients to optimise TEER outcomes. The COAPT-like profile is defined by key echocardiographic and clinical factors: LV size and function, RV function, tricuspid regurgitation, pulmonary hypertension, and haemodynamic impairment108. A single exclusion criterion is sufficient to classify a patient as having a non-COAPT-like profile (Table 3).
While the underlying mechanisms of A-SMR have been previously discussed, increasing interest has also focused on therapeutic options and predictors of outcome in this subgroup. Real-world evidence from the EuroSMR registry, which included 1,608 patients undergoing TEER for SMR, identified 126 (7.8%) with A-SMR, demonstrating its prevalence and the feasibility of TEER in this subgroup, with 2-year survival at 70.4% (similar across patients with A-SMR, non-A-SMR, and V-SMR) and procedural success (MR ≤2+ at discharge) at 87.2%32. Apart from NYHA Class IV symptoms, RV dysfunction also emerged as a significant independent predictor of 2-year mortality (p=0.014). The recent MITRA-PRO registry assessed the prognostic impact of residual MR in patients with A-SMR undergoing TEER. Procedural success was comparable between patients with A-SMR and V-SMR109. However, A-SMR patients demonstrated superior 1-year survival, even in the presence of significant residual MR. Notably, mild residual MR was associated with lower all-cause mortality (6.6% vs 10.3%) and rehospitalisation rates (29.1% vs 46.2%) compared with V-SMR. Residual MR emerged as an independent predictor of mortality in V-SMR, but not in A-SMR, suggesting a greater haemodynamic tolerance in the latter group. These findings highlight the need to minimise residual MR in V-SMR to improve outcomes, while A-SMR patients tolerate residual MR better at 1 year. A post hoc analysis of the MATTERHORN trial in A-SMR patients showed that TEER remains effective in those with preserved LVEF and atrial-driven pathology, highlighting its value in HFpEF and AF-related MR110. Isolated annular dilation was linked to better outcomes, while complex leaflet tethering was associated with poorer results. A postprocedural mean MV gradient <5 mmHg emerged as a key predictor of success. These findings stress the importance of detailed preprocedural imaging to differentiate A-SMR subtypes and guide patient selection.
Recent evidence highlights that extravalvular cardiac involvement is a critical determinant of outcomes in patients with SMR undergoing TEER26. RV dysfunction, assessed by tricuspid annular plane systolic excursion, and pulmonary hypertension have been identified as key predictors of mortality in patients with chronic HF and moderate to severe SMR111. In a cohort of 692 HF patients with SMR, the presence of pulmonary hypertension independently increased mortality risk112. Despite RV dysfunction being a key predictor of adverse outcomes, its role in SMR is not fully understood. Similarly, studies have established the impact of LA size and function on survival113. For instance, Rossi et al reported that a larger LA area is a strong predictor of adverse outcomes in HF patients with predominantly impaired systolic function114, while Palmiero et al confirmed LA dysfunction was strongly related to worse clinical status and higher incidence of pulmonary hypertension and RV dysfunction113. Notably, when patients were grouped by the extent of cardiac involvement, more advanced stages, such as RV pressure/volume overload or biventricular failure, demonstrated a stronger association with mortality compared to isolated LV or LA dysfunction.
A staging system for SMR has been proposed to stratify patients based on extramitral cardiac involvement. Stolz et al proposed a four-stage model for SMR patients with HF undergoing TEER, with stage 3 (RV pressure/volume overload) and stage 4 (biventricular failure) associated with significantly higher 2-year mortality rates40. Their findings showed a progressive, stage-dependent increase in mortality and worsening HF symptoms during follow-up. Similarly, Singh et al categorised SMR patients into four groups based on echocardiographic findings, identifying group 3 (tricuspid regurgitation and pulmonary artery involvement) and group 4 (RV dysfunction) as the strongest predictors of all-cause mortality115. Notably, extramitral valvular cardiac involvement, beyond LV dysfunction, was present in most patients with significant SMR. The Central illustration highlights the predictors of outcomes in patients with SMR and HF undergoing TEER.
Godino et al investigated the role of TEER in advanced HF patients with SMR awaiting heart transplantation as part of the MitraBridge registry. The study included 119 patients with a mean age of 58 years and severely reduced LVEF (mean LVEF of 26%)116. At 1 year, two-thirds of patients remained free from major adverse events, including death, HFH, LV assist device implantation, or heart transplantation. Notably, 23% of patients experienced sufficient clinical improvement to no longer meet the criteria for heart transplantation. Approximately 80% of patients in the MitraBridge registry would have been ineligible for TEER based on the COAPT Trial criteria but were more comparable to the MITRA-FR cohort because of severely dilated LV and proportionate MR. Despite this, MitraBridge achieved superior outcomes in reducing HFH at 1 year (30% vs 48.7% in MITRA-FR), highlighting the clinical benefit of MitraClip combined with GDMT in a younger population116. These findings support the refinement of the criteria for MitraClip therapy in advanced HF patients with significant MR who are awaiting heart transplantation.
Although many elderly patients are often ineligible for surgery because of advanced age, they stand to benefit significantly from TEER117. Scandura et al evaluated the outcomes of MitraClip implantation in high-risk patients with moderate-to-severe or severe MR, nearly half of whom were aged 75 years or older. The study demonstrated that MitraClip was safe, effective, and feasible across age groups, with similar hospital stays, 1-year outcomes, and rehospitalisation rates. Younger patients (<75 years) exhibited greater reverse LV remodelling, indicating an age-related difference in response, while both groups showed significant functional improvements80. These findings, along with data from other registries, underscore the importance of evaluating long-term outcomes in elderly patients, who represent a substantial proportion of those undergoing TEER74.
Lastly, several scoring systems have been developed to predict outcomes in patients undergoing TEER for SMR. These scores integrate clinical, anatomical, and procedural factors to aid in patient selection and risk stratification (Table 4). The MitraScore is an 8-item algorithm, that incorporates clinical factors such as age, anaemia, renal dysfunction, and LVEF to estimate mortality risk, with better predictive accuracy than traditional surgical scores like the European System for Cardiac Operative Risk Evaluation (EuroSCORE) II and the Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) score118. The EuroSMR score, an artificial intelligence-derived model, predicts 1-year mortality and functional outcomes by integrating clinical, echocardiographic, and GDMT parameters119. It offers high accuracy for identifying extreme-risk patients unlikely to benefit from TEER. Additionally, the COAPT risk score focuses on short-term outcomes by incorporating MR severity, renal function, and pulmonary pressures to stratify patients into risk categories120. While traditional surgical scores are less effective for TEER-specific predictions, these newer models provide tailored insights to optimise patient selection and procedural planning for TEER.
Table 3. COAPT criteria for TEER eligibility and patient selection.
| Category | Criteria | Rationale |
|---|---|---|
| Mitral regurgitation | Severe SMR (EROA ≥0.30 cm², regurgitant volume ≥45 mL/beat) | Ensures significant MR burden, which is required to derive haemodynamic and symptomatic benefit from TEER |
| Left ventricular function | LVEF 20-50% | Avoids extremes of LV function where advanced remodelling or preserved ejection fraction reduces the efficacy of TEER |
| Left ventricular dimensions | LVESD ≤70 mm | Patients with excessive LV dilation (>70 mm) are less likely to benefit because of irreversible LV remodelling |
| Symptomatic status | NYHA Functional Class II-IV despite ≥30 days of optimised GDMT | Reflects persistent symptoms and functional limitations despite standard care, highlighting a need for advanced intervention |
| Heart failure burden | ≥1 HF hospitalisation in the past 12 months or elevated biomarkers (BNP ≥300 pg/mL or NT-proBNP ≥1,500 pg/mL) | High HF burden predicts the benefit of MR reduction in reducing HF hospitalisations |
| Pulmonary hypertension | Pulmonary artery systolic pressure ≤70 mmHg | Severe pulmonary hypertension is associated with poor outcomes due to advanced RV dysfunction and increased procedural risk |
| Right ventricular function | No severe RV dysfunction (TAPSE >1.5 cm) | Preserved RV function is essential to support improved haemodynamics post-TEER |
| Mitral valve anatomy | Favourable anatomy (adequate leaflet coaptation, minimal tethering, no significant calcification), MV area >4.0 cm2 | Optimal leaflet morphology ensures procedural success and durability of MR reduction |
| Surgical risk | Prohibitive or high risk for mitral valve surgery | TEER is most beneficial for patients deemed at high risk or unsuitable for surgery, as it avoids high perioperative risks |
| BNP: B-type natriuretic peptide; COAPT: Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation; EROA: effective regurgitant orifice area; GDMT: guideline-directed medical therapy; HF: heart failure; LV: left ventricle; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic diameter; MR: mitral regurgitation; MV: mitral valve; NT-proBNP: N-terminal pro-B-type natriuretic peptide; NYHA: New York Heart Association; RV: right ventricle; SMR: secondary mitral regurgitation; TAPSE: tricuspid annular plane systolic excursion; TEER: transcatheter edge-to-edge repair | ||
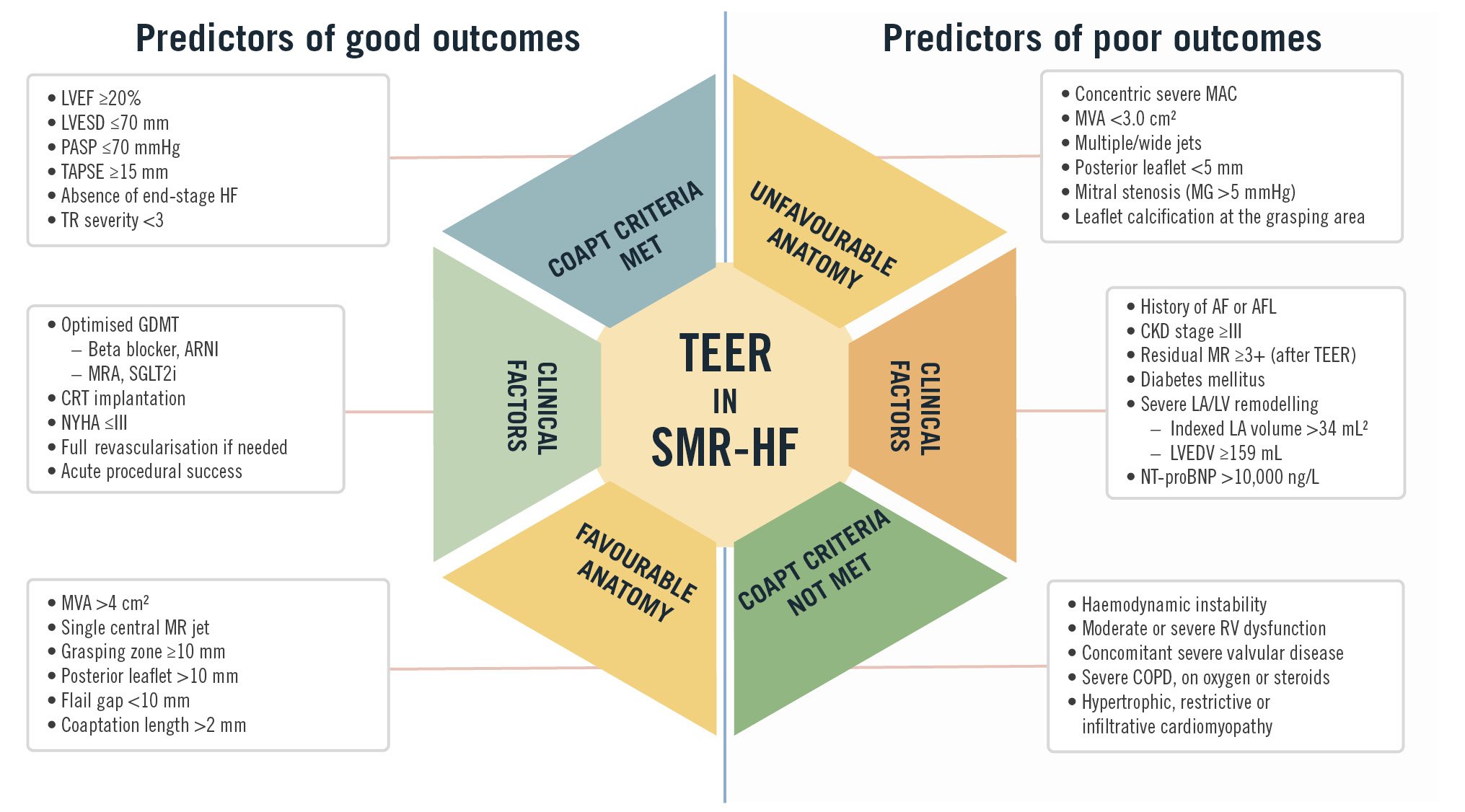
Central illustration. Predictors of outcomes in TEER for secondary mitral regurgitation and heart failure. This figure summarises the clinical and anatomical predictors of good and poor outcomes in TEER for SMR in HF. Favourable factors include adherence to COAPT Trial criteria, optimised GDMT, preserved RV function, and favourable mitral valve anatomy. AF: atrial fibrillation; AFL: atrial flutter; ARNI: angiotensin receptor-neprilysin inhibitor; CKD: chronic kidney disease; COAPT: Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation; COPD: chronic obstructive pulmonary disease; CRT: cardiac resynchronisation therapy; GDMT: guideline-directed medical therapy; HF: heart failure; LA: left atrial; LV: left ventricular; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic diameter; MAC: mitral annular calcification; MG: mean gradient; MR: mitral regurgitation; MRA: mineralocorticoid receptor antagonist; MVA: mitral valve area; NT-proBNP: N-terminal pro B-type natriuretic peptide; NYHA: New York Heart Association; PASP: pulmonary artery systolic pressure; RV: right ventricular; SGLT2i: sodium-glucose cotransporter-2 inhibitor; SMR: secondary mitral regurgitation; TAPSE: tricuspid annular plane systolic excursion; TEER: transcatheter edge-to-edge repair; TR: tricuspid regurgitation
Novel TEER devices and future directions
The field of TEER continues to expand with the development of innovative devices aimed at addressing unmet clinical needs in MR management. The DragonFly transcatheter valve repair system (Valgen Medtech) has shown promise in early studies, demonstrating high procedural success rates and significant reductions in MR severity, with 85.6% of patients achieving residual MR ≤1+ at discharge and improved NYHA Functional Class at 1-year follow-up121. Another emerging device, the NovoClasp system (Enlight Medical) is designed for intricate MV anatomies, offering high technical success rates and improved coaptation for complex MR cases122. Additionally, the GeminiOne system, developed by Peijia Medical, inspired by fourth-generation TEER technology, incorporates independent leaflet grasping and multiple clamp sizes to adapt to variable anatomies, making it particularly suitable for high-risk patients in low-income settings where cost-effectiveness is critical123. The Annular Reduction by Cinching With TEER in the Commissure (ARCTIC) procedure combines TEER with transcatheter MV replacement using the SAPIEN 3 (Edwards Lifesciences) valve platform to address challenging cases of mitral annular calcification and large annuli, preventing valve embolisation and paravalvular leaks124.
Ongoing clinical trials are poised to refine patient selection and validate the expanding role of TEER. The Transcatheter Mitral Valve Repair for Inotrope Dependent Cardiogenic Shock (MINOS; ClinicalTrials.gov: NCT05298124) trial is a randomised trial evaluating TEER’s safety and efficacy in patients with SMR and inotrope-dependent cardiogenic shock, a high-risk group currently lacking guideline recommendation. Meanwhile, the MitraClip REPAIR MR study (NCT04198870), is evaluating MitraClip TEER with contemporary surgical repair in moderate surgical risk patients with severe PMR, and the PRIMARY trial (NCT05051033) is comparing TEER and surgical repair in PMR patients older than 60 years, irrespective of the surgical risk. In the context of atrial SMR, the ongoing Treatment of Functional Mitral Regurgitation in Patients With Atrial Fibrillation (CAMERA-Pilot; NCT05846412) trial seeks to determine whether rhythm control or TEER should be prioritised in patients with concomitant AF and SMR, a key area of clinical uncertainty. Device comparison trials are also underway. The ongoing pivotal Edwards PASCAL CLASP IID/IIF Pivotal Clinical Trial (CLASP IID/IIF; NCT03706833) randomised trial is evaluating the safety and effectiveness of the PASCAL compared to the MitraClip in patients with SMR and HF with LV dysfunction, while the Efficacy of MitraCLip Vs. PASCAL for the TrEAtment of MitraL REgurgiTation in an All-comer Population study (LEAFLET I; NCT06634121) is evaluating the potential differences in procedural outcomes between MitraClip and PASCAL devices for treating MR in an “all-comer” population.
Conclusions
TEER has emerged as a transformative therapy for patients with SMR in HFrEF, providing significant reductions in mortality and HFH in appropriately selected patients. Over the past decade, advancements in imaging, device technology, and clinical evidence have firmly established TEER as a guideline-recommended treatment for patients with symptomatic SMR refractory to GDMT. Patient selection remains a critical determinant of TEER success, necessitating the careful integration of echocardiographic parameters, clinical staging systems and multidisciplinary Heart Team discussions to identify candidates most likely to benefit and optimise outcomes. While TEER has demonstrated robust safety and efficacy, ongoing research is essential to refine its application in populations with limited evidence, such as those with atrial SMR, moderate SMR, and advanced HF. Future randomised trials are crucial to delineating the role of TEER in COAPT-ineligible patients, including those with moderate SMR, asymptomatic severe SMR, SMR with HFmrEF/HFpEF, and in defining the optimal role of TEER within the broader landscape of HF management.
Acknowledgments
Dr Rodés-Cabau holds the Research Chair "Fondation Famille Jacques Larivière" for the Development of Structural Heart Interventions (Laval University, Quebec City, Canada).
Conflict of interest statement
J. Rodés-Cabau has received institutional research grants and consultant/speaker fees from Edwards Lifesciences, Abbott, and V-Wave. W.T. Abraham receives personal fees and is a shareholder of V-Wave. The other authors have no potential conflicts of interest with respect to the content of this article to declare.

