Cory:
Unlock Your AI Assistant Now!
A 79-year-old male was suffering refractory angina (daily episodes, Canadian Cardiovascular Society [CCS] class III) despite guideline-directed antianginal medical therapy including four drug classes (beta blocker, long-acting nitrate, nicorandil, ranolazine) and previous coronary artery revascularisation, and with no further suitable arterial revascularisation options after failed coronary artery bypass grafting and stenting. He was considered for a coronary sinus Reducer (Shockwave Reducer [Shockwave Medical]) implant after a multidisciplinary team discussion.
The procedure was complicated by proximal migration of the Reducer with entrapment of the delivery guide sheath at the neck of the device. Eventually, the guide sheath spontaneously disengaged, and the Reducer was anchored in the proximal coronary sinus with intentional loss of its shape, responsible for the therapeutic effect. A second Reducer was implanted distally (Moving image 1). According to institutional protocol, the patient had been on single antiplatelet therapy prior to the procedure, and dual antiplatelet therapy was planned for 6 months thereafter.
Given the complex procedure, a cardiac computed tomography (CT) at 3 months (photon-counting CT; Naeotom Alpha [Siemens Healthineers]) revealed low-attenuation material attached to the struts of both Reducers, likely representing non-occlusive thrombosis (Figure 1A-Figure 1B-Figure 1C-Figure 1D-Figure 1E-Figure 1F-Figure 1G), despite adequate parenteral anticoagulation during the procedure, as confirmed by regular monitoring of the activated clotting time above 250 seconds. Coronary sinus thrombosis is very rare and, to our knowledge, has not previously been reported following Reducer implantation1. Although not certain, it is possible that the implantation of a double device might have facilitated the thrombotic process.
Anticoagulation therapy was initiated (apixaban 5 mg bd) in combination with aspirin, and clopidogrel was discontinued. A decline in renal function precluded further CT imaging. After 6 months, invasive angiography with minimal contrast volume, supplemented by intravascular ultrasound and optical coherence tomography, confirmed patency of the sinus and demonstrated fibrous tissue within the struts, likely representing fibrotic evolution of the prior thrombosis (Figure 1H-Figure 1I-Figure 1J-Figure 1K-Figure 1L-Figure 1M). Consequently, apixaban was discontinued, and single antiplatelet therapy was maintained. At the last available follow-up (12 months after the procedure), the patient reported a marked symptomatic improvement, with a transition from CCS class III to class I whilst remaining on the same antianginal medical therapy.
In summary, this is the first case demonstrating the feasibility of a double Reducer implant while also showing that coronary sinus thrombosis may complicate the procedure. Moreover, it highlights that cardiac CT can detect sinus thrombosis and that intravascular imaging of the coronary sinus is also feasible.
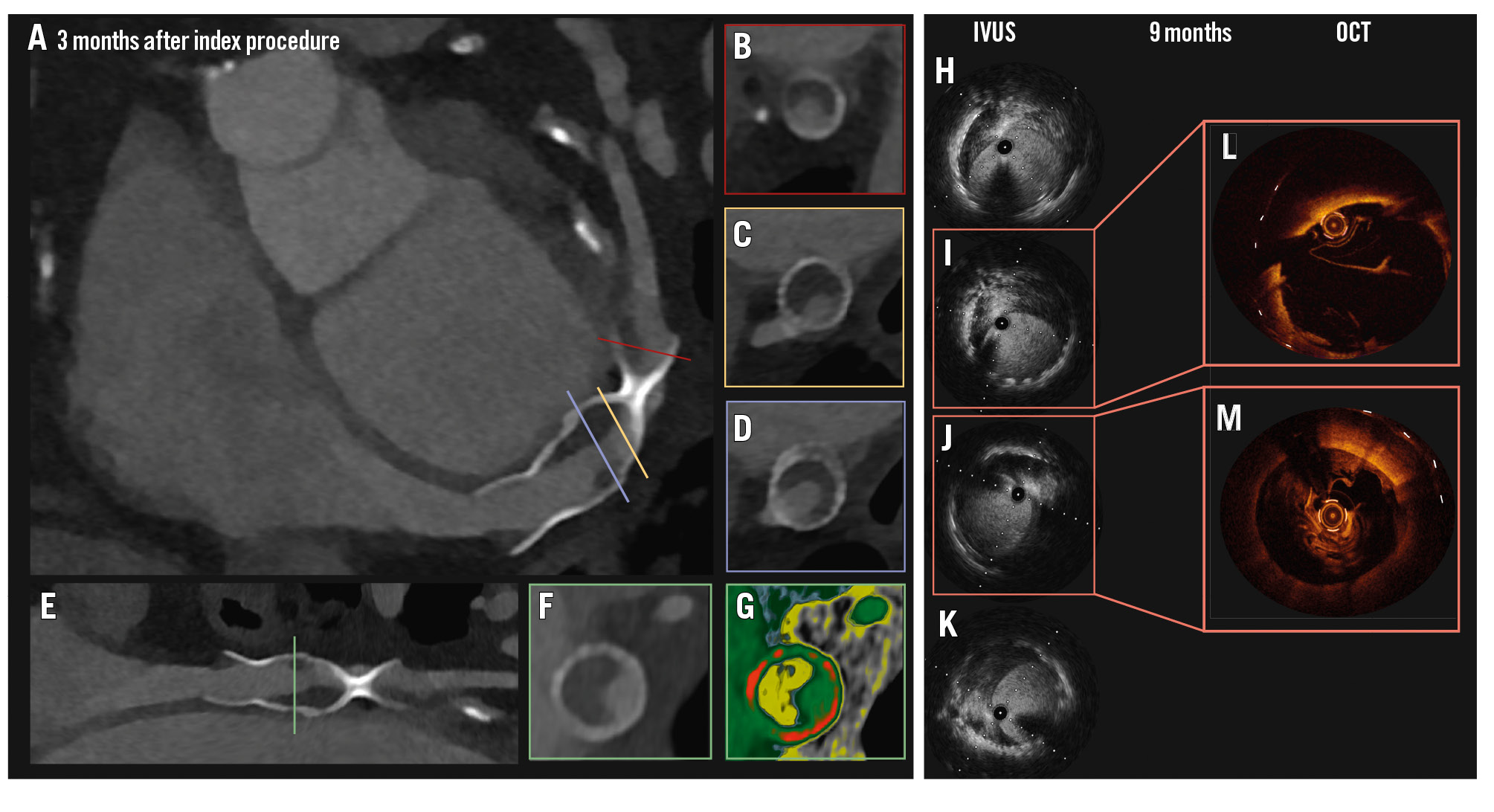
Figure 1. Coronary sinus thrombosis evolution. PCCT at 3 months (A, E) revealed low-attenuation material adherent to the struts of both Reducers, in keeping with thrombosis. Cross-sectional views (B, C, D, F) further illustrate low-attenuation material, likely representing thrombosis. A colour-coded reconstruction (Hounsfield unit attenuation) is shown in (G). IVUS (H, I, J, K) and OCT (L, M) after 6 months showed fibrotic tissue stratification inside the Reducers, likely representing organisation and chronification of previous thrombosis. IVUS: intravascular ultrasound; OCT: optical coherence tomography; PCCT: photon-counting computed tomography
Funding
This work is supported by a European Association of Cardiovascular Imaging Research Grant (2024, APP000108210), British Heart Foundation grants (FS/CRTF/23/24460), and the Onassis Foundation.
Conflict of interest statement
G.L. De Maria reports to have received speaker fees from Shockwave Medical and Abbott; consultancy fees from CorFlow; and institutional research grants from Medtronic, Terumo, and OpSens over the past 36 months. R.A. Kotronias reports honoraria from Abbott and Amarin. J.P. Langrish reports speaker fees from Boston Scientific, Abbott, and Shockwave Medical, over the prior 36 months. L. Portolan has no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Coronary sinus Reducer implantation procedural summary.

