Cory:
Unlock Your AI Assistant Now!
Abstract
Background: Transcatheter heart valve (THV) deformation after transcatheter aortic valve implantation (TAVI) using the self-expanding ACURATE platform has been associated with impaired clinical outcomes in a randomised trial. Procedural factors associated with THV deformation remain incompletely understood.
Aims: We aimed to investigate procedural factors associated with valve underexpansion, mainly derived from preprocedural computed tomography angiography (CTA) imaging.
Methods: A single-centre, retrospective, observational study was carried out, including patients who underwent TAVI with an ACURATE THV between January 2014 and December 2022, with available CTA and procedural fluoroscopy. Aortic calcium volume was quantified using 3mensio software. Fluoroscopy was used to determine valve frame underexpansion.
Results: Of 3,027 patients, 480 were eligible (74 [15.4%] with an underexpanded and 406 [84.6%] with an expanded ACURATE THV). There were no differences regarding baseline clinical and procedural characteristics. Preprocedural CTA assessment showed more calcium at the annulus level in underexpanded versus expanded THVs (60.3 [interquartile range [IQR] 21.3; 135.2] mm3 vs 45.3 [IQR 15.8; 96.1] mm3, respectively; p=0.042), while post-dilatation was less frequently performed in underexpanded compared to expanded THVs (44.6% vs 64.8%, respectively; p=0.001). Multivariable regression analysis revealed that annulus calcium volume (odds ratio [OR] 2.333, 95% confidence interval [CI]: 1.331-4.089; p=0.003) and post-dilatation (OR 0.350, 95% CI: 0.203-0.602; p<0.001) were significantly associated with underexpanded THVs. Sensitivity analysis using annulus calcium volume as a dichotomised variable (>54 mm3) confirmed the significant association with valve frame underexpansion (OR 2.38, 95% CI: 1.37-4.19; p=0.002).
Conclusions: Annular calcium volume was shown to be associated with underexpanded ACURATE THVs, while post-dilatation may reduce valve deformation.
Editorial note
On 28 May 2025, the manufacturer announced the global discontinuation of sales of its ACURATE neo2 and ACURATE Prime aortic valve systems. The following article, submitted and accepted prior to the market withdrawal, discusses clinical experience with this device. Though no longer relevant for current practice, the Editorial Board believes it is important to document these findings in the interest of transparency and completeness of the scientific record. Accordingly, we publish this work with acknowledgement of the device’s discontinued status.
Transcatheter aortic valve implantation (TAVI) has become the standard procedure for patients with severe calcific aortic valve stenosis1, and its indication has now been extended to younger and lower-risk patients234.
Self-expanding supra-annular transcatheter heart valves (THVs) were shown to provide favourable haemodynamics due to their configuration when compared to balloon-expandable intra-annular THVs5678, albeit with an increased risk of pacemaker need after TAVI910 and the disadvantage of sometimes intricate coronary access after valve implantation1112. However, the self-expanding supra-annular ACURATE (Boston Scientific) THV platform has the advantage of facilitating coronary cannulation after implantation due to the wide axial stabilisation arches and the absence of a continuous tall inner frame reaching beyond the coronary ostia. Furthermore, the ACURATE THV was shown to exhibit a comparably low risk of pacemaker implantation owing to the reduced radial strength of its nitinol frame, thereby mitigating compressive forces to the conduction system131415.
In contrast to these findings, the relatively lower radial strength of the valve might increase the risk of valve underexpansion and its associated clinical risks, as previously described, compared with fully-expanded THVs16. The mechanism behind this phenomenon is incompletely understood to date, but it has been hypothesised that underexpanded THVs augment turbulent flow and stasis in the neosinus because of inefficient leaflet functionality, including the presence of pinwheeling. The result might be increased shear stress, creating leaflet fissures, and microthrombus formation, with consequently higher risks of stroke, myocardial infarction, and probably early degeneration of the THV1718. Despite the immediate and fundamental investigation of this association after release of the ACURATE IDE trial16, with accompanying data from European centres supporting the association between underexpansion of the ACURATE neo/neo2 and impaired clinical outcome19, causal inference remains to be established.
Against this background, we aimed to investigate the procedural factors associated with underexpansion of the ACURATE neo/neo2 in a large clinical database of real-world cases.
Methods
Study population and endpoints
The current study included patients from a single high-volume centre in Germany (German Heart Center, Munich) who underwent successful TAVI using the ACURATE neo or neo2 devices after multidisciplinary Heart Team evaluation, between January 2014 and December 2022. All patients with available high-quality computed tomography angiography (CTA) for TAVI were included in the present study. Patients were treated according to local standards, and the decision regarding THV type was at the discretion of the operators.
The study was performed based on the principles of the Declaration of Helsinki. All patients provided written informed consent. Ethical approval was obtained by the ethical committee from the Technical University Munich under the registry OBSERVTAVI (525/17). CTA measurements were performed and recorded in a specific database before THV implantation. Baseline clinical characteristics, procedural characteristics, and laboratory values were entered into a customised database. For Valve Academic Research Consortium 3 (VARC-3)-defined clinical outcomes20, in-hospital and discharge follow-up was monitored and registered. The composite endpoint including all-cause mortality, stroke, and congestive heart failure rehospitalisation at 1 year was also recorded. Follow-up was performed via telephone contact, hospital visit, or follow-up letter. Electrocardiograms and biomarkers regarding periprocedural myocardial infarction after TAVI were also analysed in the underexpanded THV population according to VARC-3 definitions.
THV underexpansion analysis
For the purpose of the study, the analysis of valve underexpansion was performed using fluoroscopic imaging by an independent observer at the same centre. Angiographic studies of the TAVI procedure were retrospectively reviewed, using the latest fluoroscopic acquisition in which the degree of frame expansion could be assessed. Valve midframe underexpansion was assessed on post-implantation fluoroscopic images in an imaging plane providing the least parallax view where at least two of the three posts of the implanted THV were considered parallel to each other.
For reference, a box was drawn, delimited at the top by the lower part of the valve posts, at the bottom by the lower edge of the valve crown, and at the sides by the lateral edge of the crown portion of the valve. Once the frame was drawn, the same frame was duplicated and inserted below the first frame, and two more frames were inserted above the reference frame. Three parallel lines were drawn across the middle of the three valve posts, and the degree of parallelism of the lines was assessed in the last box, at the bottom. If two of the lines crossed in the lower box, the valve was considered to be underexpanded (Central illustration A). If none of the lines crossed, the valve was considered to be properly expanded (Central illustration C).
Computed tomography angiography analysis
All contrast-enhanced CTA studies were evaluated using 3mensio software (Pie Medical Imaging) to assess the size of the aortic valve, the level and the distribution of valvular calcification load in cubic millimetres (mm3). Aortic valve and valvular calcification measurements were obtained according to a previous publication21. In brief, the device landing zone calcium volume (DLZ-CV) was measured semiautomatically within a prespecified region of interest (above the level of the commissures including the leaflets and the left ventricular outflow tract 5 mm below the annular plane) using a scan-specific individual threshold derived from the mean attenuation of the ascending aorta plus 4 standard deviations and an additional volume filter with a threshold of 5 mm322. Calcium volume measurements were determined for the aortic valve (from the basal plane to above the commissures), aortic annulus (3 mm above the basal plane and 2 mm below the basal plane) and the left ventricular outflow tract (from the basal plane to 5 mm below it). Calcification was also measured separately for each cusp, and values were obtained in mm3, according to a previous publication where the calcium volume was validated against the standard Agatston unit21. Aortic valve measurements were obtained 0.5 mm below the basal plane to determine maximal, minimal, and mean diameter, perimeter, area, and perimeter-derived mean diameter.
Aims and endpoints
The aim of the current study was to investigate procedural factors associated with valve underexpansion, mainly derived from CTA imaging. Composite (all-cause mortality, stroke, and congestive heart failure rehospitalisation) and isolated clinical endpoints at 1 year were also evaluated. VARC-3 definitions were applied to describe procedural and follow-up outcomes.
Statistical analysis
Categorical variables are summarised using frequencies and proportions and were compared using the chi-square test. Continuous data were tested for normality with the Shapiro-Wilk test and are summarised using mean (±standard deviation) or median (interquartile range [IQR]) depending on data distribution. Differences between groups were evaluated using the Student’s t-test or Mann-Whitney U test for continuous variables as appropriate and the chi-square test or Fisher’s exact test for categorical variables.
Logistic binary regression analysis was performed to fit a univariate model for the association between calcification volume and valve underexpansion. Subsequently, receiver operating characteristic (ROC) curve analysis was performed to find a binary cutoff for the calcification volume best associated with valve underexpansion (Youden index). Adjusted multivariable analysis was performed for variables significantly associated with valve expansion by univariate analysis and those included mean aortic gradient prior to TAVI, total annulus calcification (as a continuous parameter), annulus maximum diameter, and post-dilatation. Factors inducing significant collinearity such as aortic valve orifice area and individual components of annulus calcification (at the right, left, and non-coronary cusps) were omitted to avoid model overfitting. Sensitivity analysis was performed using total annulus calcification as a binary variable after dichotomisation according to the previously established cutoff (Youden index).
Statistical analysis was performed using SPSS Statistics, Version 29 (IBM), JMP Pro, version 17.02 (SAS Institute) and RStudio (Posit Software, PBC) with R software, version 4.1 (R Foundation for Statistical Computing).
Results
Baseline and procedural characteristics
A total of 3,207 patients underwent TAVI between January 2014 and December 2022. Of these cases, 627 patients received an ACURATE neo or neo2 THV platform. From those, 480 cases were eligible (ACURATE neo=351, ACURATE neo2=129) with sufficient fluoroscopic and high-quality CTA imaging allowing for valve expansion analysis as previously described. After valve deployment, 135 cases (28.1%) were identified as having an underexpanded THV and 315 (65.6%) as having an expanded THV; in 30 cases (6.3%), it was initially impossible to determine valve expansion because of a lack of proper fluoroscopic imaging to evaluate parallelism of the posts. From the study cohort (480 patients) after post-dilatation, 74 (15.4%) were identified as having underexpanded valves and 406 (84.6%) as having well-expanded valves (Figure 1).
Baseline clinical, echocardiographic, and CTA characteristics are described in Table 1. The patients’ median age was 82 (IQR 78; 85) years, 57.9% were female, and the median European System for Cardiac Operative Risk Evaluation (EuroSCORE) II was 4.26 (IQR 2.58; 7.36). Patients with underexpanded versus expanded valves showed similar echocardiographic parameters, except their significantly higher mean aortic gradients prior to TAVI (all=41 [IQR 32; 48] mmHg, underexpanded=44 [IQR 36; 52] mmHg vs expanded=41 [IQR 32; 47] mmHg; p=0.032). CTA parameters showed no significant differences regarding overall valvular calcium volume (all=553.1 [IQR 299.1; 889.6] mm3, underexpanded=586.5 [IQR 298.8; 998.3] mm3 vs expanded=543.5 [IQR 298.2; 883.2] mm3; p=0.251), left ventricular outflow tract calcium volume (all=0.0 [IQR 0.0; 29.4] mm3, underexpanded=2.5 [IQR 0.0; 37.6] mm3 vs expanded=0.0 [IQR 0.0; 27.2] mm3; p=0.321), or total aortic calcium volume (all=577.0 [IQR 310.4; 908.5] mm3, underexpanded=605.0 [IQR 333.8; 1,006.1] mm3 vs expanded=560.5 [IQR 309.3; 900.3] mm3; p=0.238). However, there was significantly greater calcium volume at the annulus level in underexpanded versus expanded valves (60.3 [IQR 21.3; 135.2] mm3 vs 45.3 [IQR 15.8; 96.1] mm3, respectively; p=0.042).
Procedural characteristics are presented in Table 2. No differences were observed regarding procedural time (all=54 [IQR 45; 65] min, underexpanded=51 [IQR 42; 62] min vs expanded=54 [IQR 45; 65] min; p=0.095), technical success (all=93.3%, underexpanded=94.6% vs expanded=93.1%; p=0.802) or predilatation (all=99.4%, underexpanded=100% vs expanded=99.3%;p>0.999). Post-dilatation was performed significantly less frequently in underexpanded compared with expanded THVs (44.6% vs 64.8%; p=0.001). No differences regarding in-hospital cardiac biomarkers were observed between underexpanded and expanded THVs (creatine kinase [CK]: 102 [IQR 70; 180] U/L vs 106 [IQR 77; 162] U/L; p=0.786; CK-myocardial band [MB]: 19.1 [IQR 15.5; 25.7] U/L vs 18.4 [IQR 14.5; 24.1] U/L; p=0.680; high-sensitivity [hs] troponin [Tn] I: 0.12 [IQR 0.08; 0.15] ng/L vs 0.17 [IQR 0.08; 1.44] ng/L for underexpanded vs expanded valves, respectively; p=0.220). After analysing all the underexpanded THVs, no cases of periprocedural myocardial infarction (MI) were observed. There were no differences regarding procedural complications between groups.
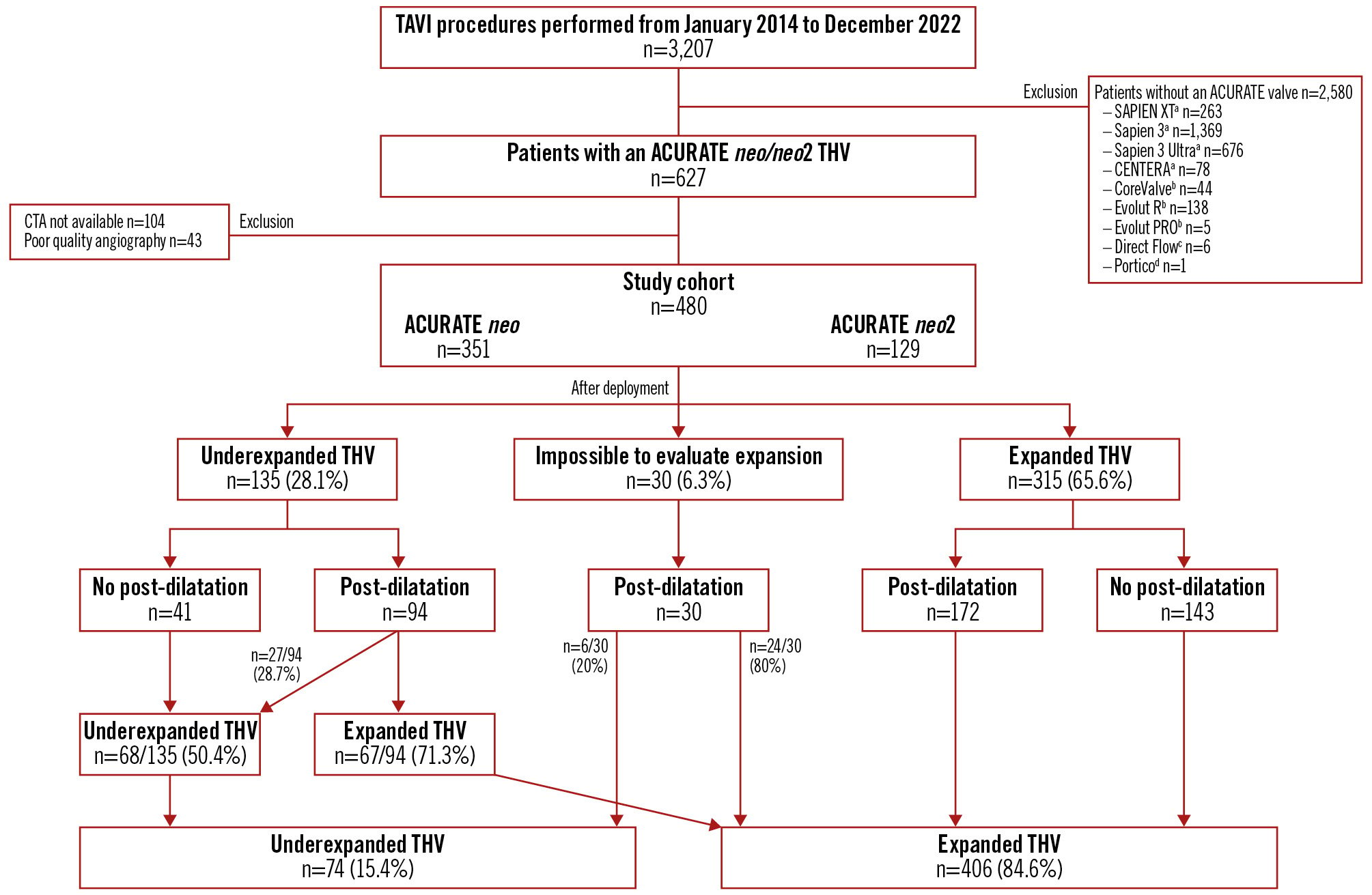
Figure 1. Flowchart. Study cohort of patients undergoing TAVI with an ACURATE neo or neo2. aBy Edwards Lifesciences; bby Medtronic; cby Direct Flow Medical; dby Abbott. CTA: computed tomography angiography; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve
Table 1. Baseline characteristics.
| All (n=480) | Underexpanded (n=74) | Expanded (n=406) | p-value | |
|---|---|---|---|---|
| Age, years | 82 [78; 85] | 83 [79; 86] | 82 [78; 85] | 0.138 |
| Female | 278 (57.9) | 46 (62.2) | 232 (57.1) | 0.445 |
| BSA, m2 | 1.82±0.20 | 1.86±0.24 | 1.82±0.19 | 0.119 |
| NYHA | 0.010 | |||
| I | 35 (7.3) | 7 (9.5) | 28 (6.9) | |
| II | 148 (30.8) | 19 (25.7) | 129 (31.8) | |
| III | 270 (56.3) | 38 (51.4) | 232 (57.1) | |
| IV | 27 (5.6) | 10 (13.5) | 17 (4.2) | |
| Hypertension | 443 (92.3) | 65 (87.8) | 378 (93.1) | 0.151 |
| Diabetes | 141 (29.4) | 20 (27.0) | 121 (29.8) | 0.679 |
| Dyslipidaemia | 373 (77.7) | 55 (74.3) | 318 (78.3) | 0.450 |
| COPD | 27 (5.8) | 8 (10.8) | 19 (4.9) | 0.057 |
| PAD | 61 (12.7) | 5 (6.8) | 56 (13.8) | 0.127 |
| Pacemaker | 46 (9.6) | 6 (8.1) | 40 (9.9) | 0.679 |
| CAD | 399 (83.1) | 60 (81.1) | 339 (83.5) | 0.614 |
| Previous PCI | 187 (39) | 28 (37.8) | 159 (39.2) | 0.897 |
| Previous MI | 49 (10.2) | 4 (5.4) | 45 (11.1) | 0.151 |
| Previous CABG | 50 (10.4) | 11 (14.9) | 39 (9.6) | 0.212 |
| Stroke/TIA | 63 (13.1) | 5 (6.8) | 58 (14.3) | 0.092 |
| History of cancer | 98 (20.4) | 11 (14.9) | 87 (21.4) | 0.214 |
| AF | 200 (41.7) | 25 (33.8) | 175 (43.1) | 0.159 |
| Dialysis | 7 (1.5) | 1 (1.4) | 6 (1.5) | >0.999 |
| EuroSCORE I | 12.93 [8.41; 20.08] | 14.86 [8.61; 20.55] | 12.63 [8.28; 20.03] | 0.273 |
| EuroSCORE II | 4.26 [2.58; 7.36] | 5.44 [2.21; 9.16] | 4.18 [2.62; 7.25] | 0.359 |
| STS-PROM score | 3.84 [2.66; 5.64] | 4.27 [2.84; 6.92] | 3.71 [2.63; 5.41] | 0.026 |
| Laboratory parameters | ||||
| Creatinine, mg/dL | 1.05 [0.89; 0.132] | 1.06 [0.90; 1.36] | 1.05 [0.89; 1.31] | 0.770 |
| Creatinine clearance, mL/min | 53.5 [41.0; 67.0] | 51.5 [38.0; 64.2] | 54.0 [41.9; 68.0] | 0.292 |
| NT-proBNP, ng/L | 1,350 [657; 3,220] | 1,120 [712; 3,232] | 1,380 [646; 3,227] | 0.555 |
| CK, U/L | 81 [59; 128] | 96 [63; 145] | 81 [59; 125] | 0.211 |
| CK-MB, U/L | 14.5 [11.8; 18.4] | 15.2 [11.6; 19.2] | 14.4 [11.8; 18.3] | 0.498 |
| Hs-troponin I, ng/L | 0.04 [0.02; 0.14] | 0.03 [0.02; 0.27] | 0.04 [0.02; 0.15] | 0.103 |
| CRP, mg/L | 1.97 [0.98; 5.33] | 2.41 [1.31; 8.67] | 1.82 [0.95; 4.98] | 0.030 |
| LDH, U/L | 206 [181; 236] | 218 [182; 241] | 205 [181; 235] | 0.198 |
| WBC, 10^9/L | 6.92 [5.93; 8.25] | 6.87 [5.89; 8.27] | 6.92 [5.94; 8.27] | 0.981 |
| Platelets, 10^9/L | 221 [188; 268] | 212 [175; 271] | 223 [191; 268] | 0.263 |
| Echocardiographic parameters | ||||
| AVA, cm2 | 0.71 [0.58; 0.84] | 0.64 [0.56; 0.80] | 0.72 [0.59; 0.85] | 0.119 |
| AVA indexed, cm2/BSA | 0.38 [0.32; 0.45] | 0.34 [0.30; 0.42] | 0.39 [0.32; 0.46] | 0.041 |
| LVEF, % | 60 [51; 60] | 60 [50; 60] | 60 [51; 60] | 0.861 |
| Aortic mean gradient, mmHg | 41 [32; 48] | 44 [36; 52] | 41 [32; 47] | 0.032 |
| Aortic maximal gradient, mmHg | 66 [54; 78] | 70 [57; 82] | 66 [54; 78] | 0.220 |
| Systolic pulmonary artery pressure, mmHg | 40 [33; 48] | 37 [29; 49] | 40 [33; 48] | 0.367 |
| Tomographic parameters | ||||
| Bicuspid | 21 (4.4) | 2 (2.7) | 19 (4.7) | 0.555 |
| Valvular calcium volume on NCC, mm3 | 229.8 [123.8; 389.9] | 232.4 [130.5; 466.2] | 229.4 [122.8; 381.5] | 0.379 |
| Valvular calcium volume on RCC, mm3 | 146.5 [67.1; 261.0] | 158.7 [66.9; 297.8] | 139.6 [66.9; 258.1] | 0.172 |
| Valvular calcium volume on LCC, mm3 | 132.3 [73.0; 257.5] | 163.3 [81.1; 262.8] | 129.5 [72.8; 258.5] | 0.237 |
| Total valvular calcium volume, mm3 | 553.1 [299.1; 889.6] | 586.5 [298.8; 998.3] | 543.5 [298.2; 883.2] | 0.251 |
| Annular calcium volume on NCC, mm3 | 15.3 [0.0; 41.5] | 18.5 [0.0; 49.0] | 14.7 [0.0; 41.1] | 0.538 |
| Annular calcium volume on RCC, mm3 | 0.0 [0.0; 16.9] | 7.8 [0.0; 49.0] | 0.0 [0.0; 16.0] | 0.001 |
| Annular calcium volume on LCC, mm3 | 11.9 [0.0; 39.6] | 20.8 [0.0; 47.2] | 10.1 [0.0; 38.2] | 0.039 |
| Total annular calcium volume, mm3 | 46.0 [16.4; 100.5] | 60.3 [21.3; 135.2] | 45.3 [15.8; 96.1] | 0.042 |
| LVOT calcium volume on NCC, mm3 | 0.0 [0.0; 0.0] | 0.0 [0.0; 1.6] | 0.0 [0.0; 0.0] | 0.167 |
| LVOT calcium volume on RCC, mm3 | 0.0 [0.0; 0.0] | 0.0 [0.0; 0.0] | 0.0 [0.0; 0.0] | 0.227 |
| LVOT calcium volume on LCC, mm3 | 0.0 [0.0; 13.0] | 0.0 [0.0; 8.6] | 0.0 [0.0; 14.2] | 0.938 |
| Total LVOT calcium volume, mm3 | 0.0 [0.0; 29.4] | 2.5 [0.0; 37.6] | 0.0 [0.0; 27.2] | 0.321 |
| Total calcium volume, mm3 | 577.0 [310.4; 908.5] | 605.0 [333.8; 1,006.1] | 560.5 [309.3; 900.3] | 0.238 |
| Calcium asymmetry | 184 (38.4) | 29 (39.2) | 155 (38.3) | 0.897 |
| Annulus minimum diameter, mm | 20.2±1.9 | 20.1±1.9 | 20.3±1.9 | 0.399 |
| Annulus maximum diameter, mm | 26.4 [24.8; 28.0] | 25.8 [24.1; 27.6] | 26.5 [24.8; 28.1] | 0.039 |
| Annulus mean diameter, mm | 23.3 [22.0; 24.7] | 23.1 [21.5; 24.3] | 23.4 [22.1; 24.8] | 0.106 |
| Annulus perimeter, mm | 74 [70; 78] | 73 [68; 77] | 74 [70; 78] | 0.094 |
| Annulus mean diameter derived from perimeter, mm | 23.5 [22.3; 24.9] | 23.2 [21.7; 24.6] | 23.5 [22.3; 24.9] | 0.094 |
| Annulus area, mm2 | 415 [373; 467] | 404 [357; 458] | 417 [375; 469] | 0.138 |
| Annulus mean diameter derived from area, mm | 23.0 [21.8; 24.4] | 22.7 [21.3; 24.1] | 23.0 [21.8; 24.4] | 0.137 |
| Eccentricity index | 0.23 [0.19; 0.27] | 0.22 [0.19; 0.26] | 0.23 [0.19; 0.28] | 0.381 |
| Sinotubular junction height, mm | 21.7 [20.0; 23.7] | 21.0 [19.3; 23.2] | 21.9 [20.0; 23.9] | 0.074 |
| Sinotubular junction width, mm | 26.3 [24.8; 28.7] | 26.0 [24.2; 28.2] | 26.5 [24.8; 28.8] | 0.118 |
| Ascending aorta width, mm | 34.0 [31.5; 36.7] | 33.2 [31.4; 36.7] | 34.0 [31.5; 36.8] | 0.338 |
| Left main height, mm | 13 [12; 15] | 13 [11; 15] | 14 [12; 16] | 0.074 |
| RCA height, mm | 17 [15; 19] | 17 [14; 19] | 17 [15; 19] | 0.503 |
| Data are presented as median [IQR], mean±SD, or number (%) as appropriate. AF: atrial fibrillation; AVA: aortic valve area; BSA: body surface area; CABG: coronary artery bypass graft; CAD: coronary artery disease; CK: creatine kinase; CK-MB: creatine kinase-myocardial band; COPD: chronic obstructive pulmonary disease; CRP: C-reactive protein; EuroSCORE: European System for Cardiac Operative Risk Evaluation; hs: high-sensitivity; IQR: interquartile range; LCC: left coronary cusp; LDH: lactate dehydrogenase; LVEF: left ventricular ejection fraction; LVOT: left ventricular outflow tract; MI: myocardial infarction; NCC: non-coronary cusp; NT-proBNP: N-terminal pro-brain natriuretic peptide; NYHA: New York Heart Association; PAD: peripheral artery disease; PCI: percutaneous coronary intervention; RCA: right coronary artery; RCC: right coronary cusp; SD: standard deviation; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality; TIA: transient ischaemic attack; WBC: white blood cell | ||||
Table 2. Procedural characteristics.
| All (n=480) | Underexpanded (n=74) | Expanded (n=406) | p-value | |
|---|---|---|---|---|
| Cerebral protection device | 9 (1.9) | 0 (0) | 9 (2.2) | 0.196 |
| Procedural time, min | 54 [45; 65] | 51 [42; 62] | 54 [45; 65] | 0.095 |
| Contrast media, mL | 150 [120; 196] | 140 [110; 182] | 150 [120; 200] | 0.075 |
| Fluoroscopy time, min | 12.7 [10.2; 16.5] | 12.2 [9.4; 16.9] | 12.8 [10.3; 16.3] | 0.381 |
| Fluoroscopy dose, cGy*cm2 | 1,885 [793; 3,077] | 2,133 [873; 3,387] | 1,842 [774; 3,044] | 0.457 |
| ACURATE | 0.032 | |||
| neo | 351 (73.1) | 62 (83.8) | 289 (71.2) | |
| neo2 | 129 (26.9) | 12 (16.2) | 117 (28.9) | |
| Size of valve implanted | 0.186 | |||
| 23 mm | 139 (29) | 28 (37.8) | 111 (27.3) | |
| 25 mm | 209 (43.5) | 29 (39.2) | 180 (44.3) | |
| 27 mm | 132 (27.5) | 17 (23.0) | 115 (28.3) | |
| THV over- or undersizing, % | –6 [–9; –3] | –6 [–8; –3] | −6 [–9; –3] | 0.664 |
| Predilatation | 477 (99.4) | 74 (100) | 403 (99.3) | >0.999 |
| Semicompliant balloon | 312 (65.4) | 51 (68.9) | 264 (65.5) | 0.550 |
| Non-compliant balloon | 165 (34.6) | 23 (31.1) | 139 (34.5) | 0.550 |
| Predilatation balloon size, mm | 23 [20; 24] | 22 [20; 23.3] | 23 [21; 24] | 0.082 |
| Balloon-to-annulus ratio | 0.96 [0.93; 0.98] | 0.95 [0.93; 0.99] | 0.96 [0.93; 0.98] | 0.433 |
| Post-dilatation | 296 (61.7) | 33 (44.6) | 263 (64.8) | 0.001 |
| Semicompliant balloon | 201 (67.9) | 25 (75.8) | 176 (66.9) | 0.280 |
| Non-compliant balloon | 95 (32.1) | 8 (24.2) | 87 (33.1) | 0.280 |
| Post-dilatation balloon size, mm | 23 [22; 24] | 23 [20.5; 24] | 23 [22; 24] | 0.593 |
| Balloon-to-annulus ratio | 0.97 [0.94; 0.99] | 0.95 [0.93; 0.98] | 0.97 [0.94; 0.99] | 0.034 |
| Technical success | 448 (93.3) | 70 (94.6) | 378 (93.1) | 0.802 |
| Tamponade | 2 (0.4) | 0 (0) | 2 (0.5) | >0.999 |
| Annulus rupture | 1 (0.2) | 0 (0) | 1 (0.2) | >0.999 |
| Conversion to surgery | 2 (0.4) | 0 (0) | 2 (0.5) | >0.999 |
| Aortic insufficiency ≥2 | 14 (2.9) | 2 (2.8) | 12 (3.0) | >0.999 |
| Mean gradient post-intervention, mmHg | 8 [6; 11] | 8 [6; 11] | 8 [6; 11] | 0.387 |
| In-hospital mortality | 1 (0.2) | 0 (0) | 1 (0.2) | >0.999 |
| Days in ICU | 1 [1; 1] | 1 [1; 1] | 1 [1; 2] | 0.010 |
| In-hospital laboratory parameters | ||||
| NT-proBNP, ng/L | 1,680 [566; 3,400] | 1,340 [566; 2,957] | 1,690 [565; 4,900] | 0.537 |
| CK, U/L | 105 [77; 162] | 102 [70; 180] | 106 [77; 162] | 0.786 |
| CK-MB, U/L | 18.4 [14.5; 24] | 19.1 [15.5; 25.7] | 18.4 [14.5; 24.1] | 0.680 |
| Hs-troponin I, ng/L | 0.13 [0.08; 0.47] | 0.12 [0.08; 0.15] | 0.17 [0.08; 1.44] | 0.220 |
| CRP, mg/L | 55.8 [33.8; 83.3] | 59.4 [37.9; 78.8] | 54.8 [33.6; 83.6] | 0.585 |
| LDH, U/L | 239 [209; 283] | 244 [205; 298] | 238 [209; 282] | 0.350 |
| WBC, 10^9/L | 10.4 [8.68; 12.5] | 9.8 [8.26; 11.13] | 10.6 [8.74; 12.63] | 0.029 |
| Platelets, 10^9/L | 197 [165; 239] | 189 [154; 226] | 198 [167; 240] | 0.126 |
| Data are presented as median [IQR] or number (%) as appropriate. CK: creatine kinase; CK-MB: creatine kinase-myocardial band; CRP: C-reactive protein; hs: high-sensitivity; ICU: intensive care unit; IQR: interquartile range; LDH: lactate dehydrogenase; NT-proBNP: N-terminal pro-brain natriuretic peptide; THV: transcatheter heart valve; WBC: white blood cell | ||||
Post-dilatation effect in THV expansion
Of the 296 patients that received a post-dilatation strategy, angiographic images before and after post-dilatation were available in 266 (89.9%) patients. Underexpanded THVs were modified to expanded THVs from 94 (35.3%) to 67 patients (25.2%) after post-dilatation. Among the 30 patients (6.3%) without fluoroscopic imaging before post-dilatation, 6 patients (20%) were identified as having underexpanded THVs, while 24 (80%) had expanded THVs after post-dilatation.
Clinical and echocardiographic outcomes at follow-up
Clinical outcomes at 1 year are shown in Supplementary Table 1. No differences between underexpanded and expanded THVs were detected regarding the composite endpoint (all-cause mortality, stroke, and congestive heart failure; 12.2% vs 11.8%;p>0.999), all-cause mortality (8.1% vs 6.4%; p=0.611), stroke (1.4% vs 1.5%;p>0.999), or congestive heart failure (5.4% vs 5.2%;p>0.999) at 1-year follow-up; mortality at the longest available follow-up (802 [413; 1,374] days) was also similar between groups (33.8% vs 27.1%; p=0.239) (Supplementary Figure 1). No significant differences between New York Heart Association (NYHA) heart failure classification were observed at 30 days or 1 year between underexpanded and expanded THVs (Figure 2). No differences regarding laboratory parameters (N-terminal pro-brain natriuretic peptide, CK, CK-MB, hsTnI) at 30 days or 1 year between underexpanded and expanded THVs were observed (Supplementary Table 2). Echocardiographic outcomes at 30 days and 1 year are summarised in Figure 3 and Supplementary Table 1. There was a significantly higher transvalvular mean gradient at 30 days in underexpanded compared to expanded THVs (8.9±4.1 mmHg vs 7.4±3.6 mmHg; p=0.006), but there was no difference at 1 year. No differences were observed regarding aortic valve area at 30 days or 1 year. Aortic insufficiency that was more than moderate was not significantly different between underexpanded and expanded THVs at 30 days (0% vs 1.0%;p>0.999) or at 1 year (5.7% vs 1.6%; p=0.172).
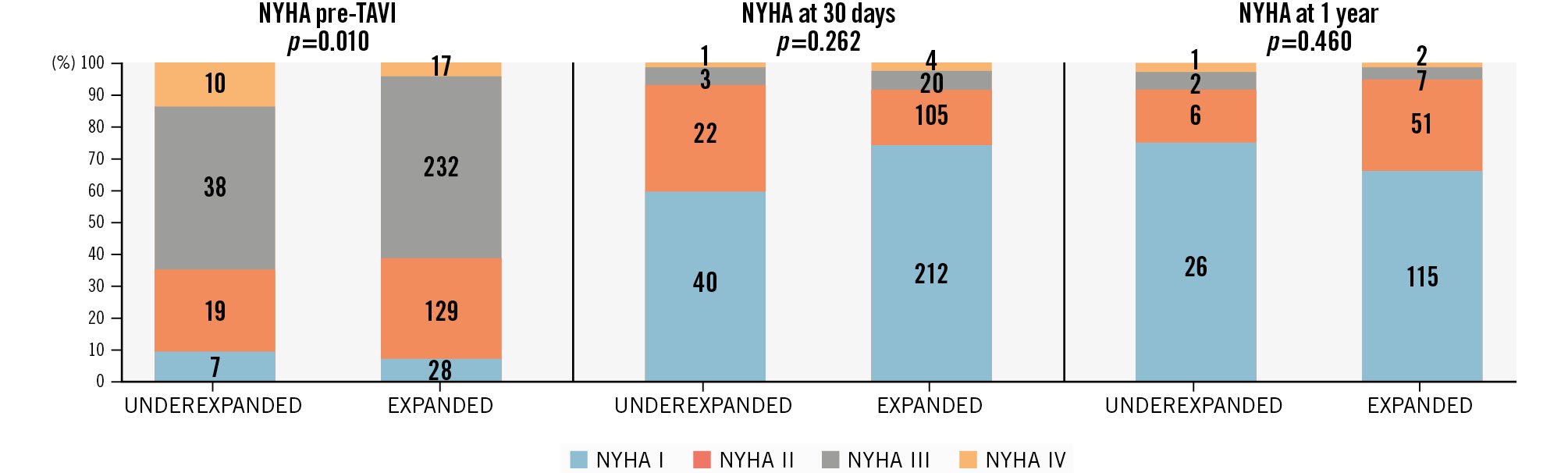
Figure 2. Heart failure symptoms. NYHA classification before, at 30 days, and at 1 year after aortic valve implantation in both groups: underexpanded and expanded transcatheter heart valves. NYHA: New York Heart Association; TAVI: transcatheter aortic valve implantation
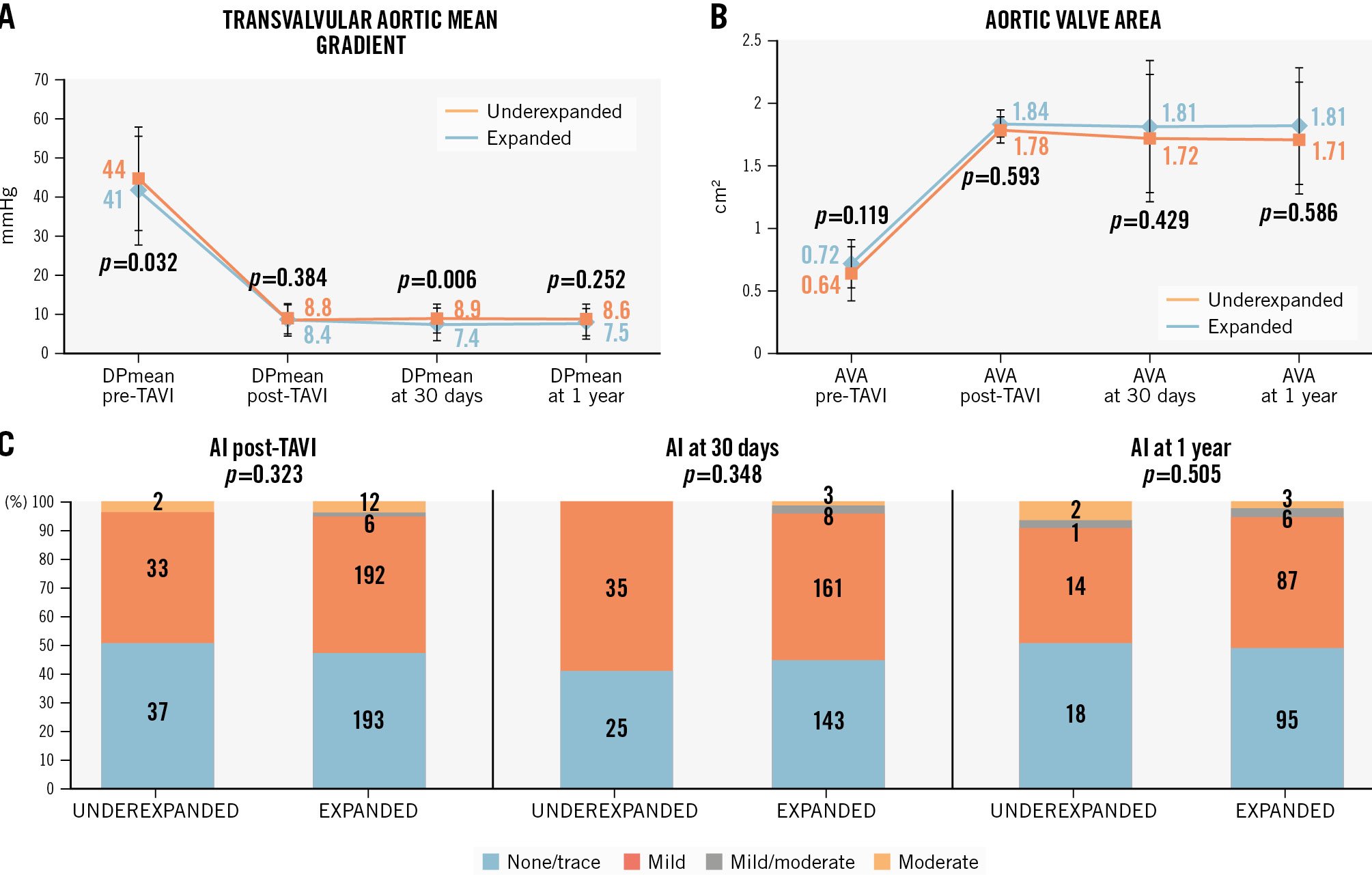
Figure 3. Echocardiographic aortic valvular parameters during follow-up (30 days and 1 year). A) Transvalvular aortic mean gradient; (B) aortic valve area; (C) aortic insufficiency. AI: aortic insufficiency; AVA: aortic valve area; DPmean: delta pressure mean gradient; TAVI: transcatheter aortic valve implantation
Factors associated with an underexpanded THV
Unadjusted binary logistic regression analysis showed that annulus calcium volume (per 1 mm3 increment of calcium volume; odds ratio [OR] 1.003, 95% confidence interval [CI]: 1.000-1.005; p=0.024), the ACURATE neo THV platform (OR 2.092, 95% CI: 1.087-4.024; p=0.027), and post-dilatation (OR 0.438, 95% CI: 0.265-0.723; p=0.001) were associated with underexpanded valves. Annulus calcium volume was also dichotomised using a cutoff value (>54 mm3) derived from ROC analysis (Supplementary Table 3), with an OR of 1.804, 95% CI: 1.094-2.975; p=0.022. After multivariable analysis (Table 3), only annulus calcium volume (per 1 mm3 of calcium volume, OR 2.333, 95% CI: 1.331-4.089; p=0.003), and post-dilatation (OR 0.350, 95% CI: 0.203-0.602; p<0.001) remained significant (Central illustration). Sensitivity analysis using annulus calcium volume as a dichotomised variable (>54 mm3) confirmed the significant association with valve frame underexpansion (OR 2.38, 95% CI: 1.37-4.19; p=0.002) (Supplementary Figure 2).
Table 3. Multivariable analysis for underexpanded valves.
| OR | 95% CI | p-value | |
|---|---|---|---|
| Transvalvular aortic mean gradient preintervention, mmHg | 1.009 | 0.990-1.029 | 0.317 |
| Annulus maximal diameter, mm | 0.896 | 0.794-1.011 | 0.071 |
| Annulus calcium volume, for a 1 mm3 increment | 2.333 | 1.331-4.089 | 0.003* |
| Post-dilatation | 0.350 | 0.203-0.602 | <0.001* |
| ACURATE neo | 1.940 | 0.986-3.815 | 0.054 |
| *Indicates statistical significance. CI: confidence interval; OR: odds ratio | |||
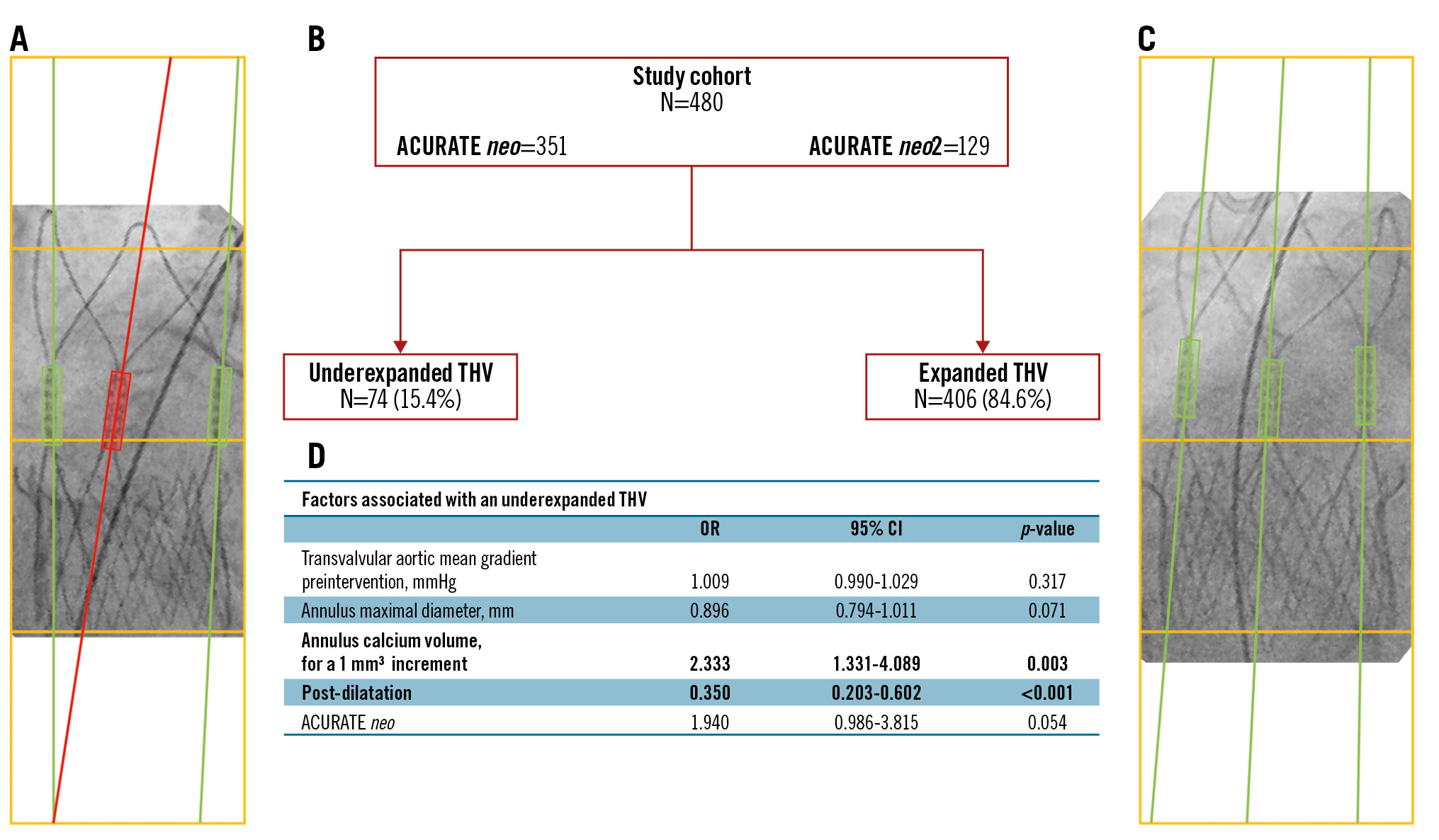
Central illustration. Factors associated with an underexpanded ACURATE transcatheter heart valve. A) ACURATE neo prosthesis with some degree of deformation (underexpanded), shown by the red line in the middle which intersects with the lateral line at the bottom within the box. B) Simple study flowchart. C) ACURATE neo prosthesis with no deformation (expanded), shown by the parallelism of the three green lines through the image. D) The factors found to be associated with THV underexpansion. CI: confidence interval; OR: odds ratio; THV: transcatheter heart valve
Discussion
This single-centre observational study sought to investigate factors associated with underexpanded ACURATE neo and ACURATE neo2 THVs in patients with severe aortic valve stenosis and an indication for TAVI. The main findings of the current study can be summarised as follows: (1) higher annular calcium volume increased the risk of underexpansion of the ACURATE neo/neo2 THV; (2) post-dilatation decreased the risk of underexpansion of the ACURATE neo/neo2 THV; and (3) 1-year mortality appeared to be numerically higher in patients with underexpanded THVs, yet without reaching statistical significance. Importantly, this study identified the relevance of annulus calcium volume evaluation when considering which ACURATE neo/neo2 THV should be selected to treat degenerative calcific aortic valve stenosis (CAVS). This finding may have relevance to determine individualised treatment approaches for patients suffering CAVS. Importantly, post-dilatation was found to be an effective measure to correct underexpanded ACURATE neo/neo2 valves, modifying the frame deformation to expanded frames in 71.3% of patients.
In the ACURATE IDE randomised controlled trial16, the ACURATE neo2 failed to meet non-inferiority for the primary endpoint of all-cause mortality, stroke, or rehospitalisation at 1 year using a Bayesian approach against a control group composed of U.S. Food and Drug Administration-approved THVs (SAPIEN 3/3 Ultra [Edwards Lifesciences] and Evolut R/PRO/PRO+/FX [Medtronic]): ACURATE neo2: 16.16%, control: 9.53%; posterior median difference 6.63% (95% Bayesian credible interval: 3.04-10.20%)16. Given the unexpected outcome of this study, an in-depth investigation into potential root causes of this failure was immediately initialised and reported16; approximately 20% of the ACURATE neo2 valves in the IDE trial were found to be underexpanded, which is higher compared to previously reported studies evaluating this valve19 and also compared with the 15.4% of underexpanded ACURATE neo/neo2 valves in our cohort. In the ACURATE IDE trial, underexpansion was associated with a 1-year composite event rate (death, stroke, and rehospitalisation) of 18.8% versus 12.4% in the adequate expansion group. In contrast, we observed a 1-year composite event rate of 12.2% in the underexpanded valve group and 11.8% in the adequately expanded valve group. While this difference was not statistically significant, a trend towards higher event rates in the underexpanded group was apparent. This may be explained by the smaller sample size in our study and the fact that it was not specifically powered to evaluate composite clinical outcomes. This trend of increased 1-year event rates in the underexpanded valve group was also observed in the individual outcomes of mortality and heart failure-related hospitalisation but not in stroke, possibly due to underdiagnosis. Nevertheless, the overall 1-year mortality rate in our cohort was comparable to that reported in previous studies23. In aggregate, these data for the first time suggest a direct link between valve frame underexpansion of the ACURATE neo/neo2, malfunctioning of the valve leaflets causing microembolism, and consequently, increased rates of ischaemic events such as myocardial infarction and stroke. Despite the seemingly intuitive pathophysiology behind the increased clinical event rates of underexpanded ACURATE neo/neo2 valves, direct evidence for the mechanistic interaction is missing to date, and careful future investigation is warranted in this regard. This proposition is also fuelled by the observation in the ACURATE IDE trial of increased ischaemic event rates after 30 days, suggesting microembolism occurring beyond this time frame and not in the periprocedural phase. This is confirmed by the absence of periprocedural MI according to VARC-3 definitions in our study.
Interestingly, midframe underexpansion of the ACURATE neo/neo2 did not affect aortic valve area or haemodynamic mean gradients at 1-year follow-up in the studied population. No major differences in heart failure symptoms or aortic insufficiency were observed between patients with underexpanded and expanded valves. Although no statistically significant differences were observed, there was a tendency towards higher mean transvalvular gradients, a smaller valve area, as well as greater functional class impairment and moderate aortic insufficiency, in patients with an underexpanded valve. These might be considered the haemodynamic consequences of underexpanded valve frames.
Our findings demonstrated that annular calcification increased the risk of prosthetic valve deformation, implying that a higher calcium burden at the aortic annulus level was associated with a greater likelihood of prosthesis deformation after implantation. Valvular calcification has previously been evaluated as a predictor of mortality21 and as a risk factor for annulus rupture24 and paravalvular regurgitation25. Furthermore, in certain CTA-based assessment models, valvular calcium has been shown to impair optimal expansion of balloon-expandable THVs2627 and also to distort self-expanding THVs at the annular level28. Until recently, the clinical relevance of detecting prosthetic valve deformation was based on the theoretical association with hypoattenuated leaflet thickening, which may in turn compromise prosthesis function and durability, while also increasing the risks of thrombosis, mortality, and hospitalisation2930. To address this, appropriate implantation strategies should be considered, including adequate predilatation, careful selection of the prosthetic valve tailored to patient-specific anatomy, guided in part by quantification of valvular calcium volume, and the use of post-dilatation when appropriate.
In this regard, we observed that post-dilatation was more frequently performed in patients with adequately expanded prostheses, suggesting that this approach should be considered in recipients of the ACURATE THV. Importantly, the decision to post-dilate should be based on angiographic confirmation of valve expansion as previously established16. In a stringent way, the company has advocated a simplified onsite assessment of valve expansion using two different c-arm angulations. Should underexpansion be detected in one of these two projections, post-dilatation is warranted. Post-dilatation of self-expanding valves has already been associated with reduced underexpansion and, consequently, with a lower incidence of leaflet thickening31, findings in clear support of the current study.
Limitations
The present study is limited by the observational nature of the investigation, selection bias of patients assigned to procedures, and it is limited to 1-year follow-up. The number of patients included in the study was not sufficient to observe a statistically significant difference in clinical events at 1 year, although there is a trend of higher events in the underexpanded valve population.
Conclusions
Annular calcium volume was shown to be associated with underexpanded ACURATE neo/neo2 THVs, and a post-dilatation strategy may reduce this valve deformation. Future studies will need to address this association in a prospective controlled way and determine whether the use of this specific THV system is reasonable for treating severely calcified aortic valve stenosis.
Impact on daily practice
Valvular deformation or underexpansion of the supra-annular ACURATE neo/neo2 self-expanding valve following transcatheter aortic valve implantation is associated with prosthesis malfunction and increased risk of clinical events. Excessive annular calcium volume is associated with a higher likelihood of underexpansion of the ACURATE prosthesis, and post-dilatation reduces the risk of underexpansion. Whether valve frame underexpansion is observed at a similar frequency in other transcatheter heart valve platforms and whether this is also associated with adverse clinical outcome needs to be studied in dedicated clinical trials.
Guest Editor
This paper was guest edited by Franz-Josef Neumann, MD, PhD; Department of Cardiology and Angiology, University Heart Center Freiburg - Bad Krozingen, Bad Krozingen, Germany.
Conflict of interest statement
H.A. Alvarez-Covarrubias received lecture fees from SIS Medical AG, LifeTech, and Edward Lifesciences, not related to the current work. Y. Taniguchi received lecture honoraria from Boston Scientific; and received information provision fees from Win International. T. Rheude received lecture fees from AstraZeneca, Abbott, SIS Medical, and Translumina; and a travel grant to the institution from Boston Scientific, not related to this work. H. Schunkert reports personal fees from Amgen, Daiichi Sankyo, MSD Sharp&Dohme GmbH, AstraZeneca, Bayer Vital, Boehringer Ingelheim, Novartis, Servier, Sanofi Aventis, and Synlab, not related to the current work. E. Xhepa reports lecture fees from AstraZeneca, Boston Scientific, and SIS Medical; and financial support from Abbott, not related to the current work. M. Joner reports personal fees from Abbott, AlchiMedics S.A.S., AstraZeneca, Biotronik, Medtronic, Recor Medical, Shockwave Medical, TRiCares, and Veryan; grants and personal fees from Boston Scientific, Cardiac Dimensions, and Edwards Lifesciences; and a grant from Infraredx, outside the submitted work. The other authors have no conflicts of interest to declare. The Guest Editor reports consultancy fees from Novartis and Meril Life Sciences; and speaker honoraria from Meril Life Sciences.
Supplementary data
To read the full content of this article, please download the PDF.

