Cory:
Unlock Your AI Assistant Now!
Over the past two decades, lipid-lowering interventions have been one of the most used and effective treatments for modifying a patient’s cardiovascular risk and plaque composition. It has also been demonstrated that the intensity of their effect matters; the lower the low-density lipoprotein cholesterol (LDL-C), the higher the reduction in risk and the more “stabilising” the plaque modifications.
In this issue of EuroIntervention, Mensink et al present the results of the FITTER study1, in which the main research question was whether evolocumab, plus statin, influences ischaemia-producing coronary plaques in a short period of time. Following a well-executed randomised clinical trial, the authors summarised the main results as follows: “... no between-group differences were found between evolocumab- and placebo-treated patients”. I agree with the authors that there was no statistically significant difference, but this does not equate to the absence of clinical relevance, since their results help to understand the developing changes in coronary plaque as induced by proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i).
The study’s main endpoints were lipid core burden index (LCBI) and fractional flow reserve (FFR). The merits of each of these variables are discussed here below.
The lipid core burden index: coronary lipid content, as measured by near-infrared spectroscopy (NIRS) intravascular ultrasound (IVUS), has been associated with an increased risk of cardiovascular events2. The main outcome variable is LCBI. LCBI is then a high-risk plaque future whose changes have been assessed by using high-intensity statin therapy (HIST) alone or in combination with PCSK9i. In the IBIS-3 study3, HIST (i.e., rosuvastatin) alone did not show a sizeable effect on LCBI after 1 year of treatment. Conversely, the PACMAN-AMI trial45 showed that the combination of HIST plus alirocumab (PCSK9i) changed the LCBI by –79.42 at 52 weeks (from 260.6; the % difference is 30.48) (Figure 1); whereas in the FITTER study, LCBI changed by –27.8 at 12 weeks (from 354.7; the % difference is 7.78) (Figure 1). Of note, the extent of these compositional changes was not followed by a change of similar magnitude in plaque size as measured by percent atheroma volume (PAV); in the former study the PAV changed by 5.2%, and in the latter, the PAV changed by 1%. Furthermore, these plaque changes should be put into context with the LDL-C reduction occurring in these patients; the LDL-C values reduced by ~60% within two weeks.
The beneficial PCSK9i effects are seen across three periods (Figure 1): first, in the blood, followed by the plaque, and finally, clinically. The chemical changes in blood are seen within 2 weeks and maintained thereafter. The compositional (lipid) changes start to be relevant by the 12-week timepoint and are probably at their best at 52 weeks, when we observed the divergence of the Kaplan-Meier curves, showing a reduction in clinical outcomes in the FOURIER study6. Thus, it is assumable that blood and plaque composition needed to change first to matter in terms of the reduction of clinical outcomes.
The fractional flow reserve: it is uncommonly used as an endpoint in lipid-lowering trials. Looking at Figure 1, the reader may understand that it is because the changes in minimum lumen area (MLA), which are the strongest determinant of FFR, are small and slow to develop. Thus, the FFR is not expected to change rapidly. In the FITTER study, the MLA did not change from baseline to 12 weeks, while in the PACMAN-AMI study, MLA increased by 0.15 mm2, which represents a 2.5% increase (Figure 1).
Taken all together, it means that the patients/lesions enrolled in the FITTER study started to get “fit” by experiencing changes in the composition of the plaque that was “converting” lipid into a different tissue type and thereby not impacting overall plaque size; this dynamic process of “substituting” tissue types has been documented in the optical coherence tomography trials, in which fibrotic tissue increased thickening of the cap while the lipid arc decreased7. Additionally, through the PACMAN-AMI trial results, it appears that the compositional changes continue to occur at a faster rate compared to the overall plaque size and a discreet change in lumen size at 52 weeks.
Unlike most clinical randomised trials evaluating coronary artery disease progression/regression, the FITTER study included more severe and advanced lesions which were ischaemia-producing (FFR range of 0.67-0.85). The investigators’ rationale for including these lesions was based on the expectation of observing a more pronounced effect. While their expectation is based on previous research reports, it may also mean that the lesions were more calcified – lesions which have been reported to be more “resistant” to undergoing changes in size in response to systemic therapies8.
Mechanistic studies using imaging advance the understanding of the evolution of atherosclerosis and its response to systemic therapies. More importantly, they provide information that may explain the clinical benefits of these therapies.
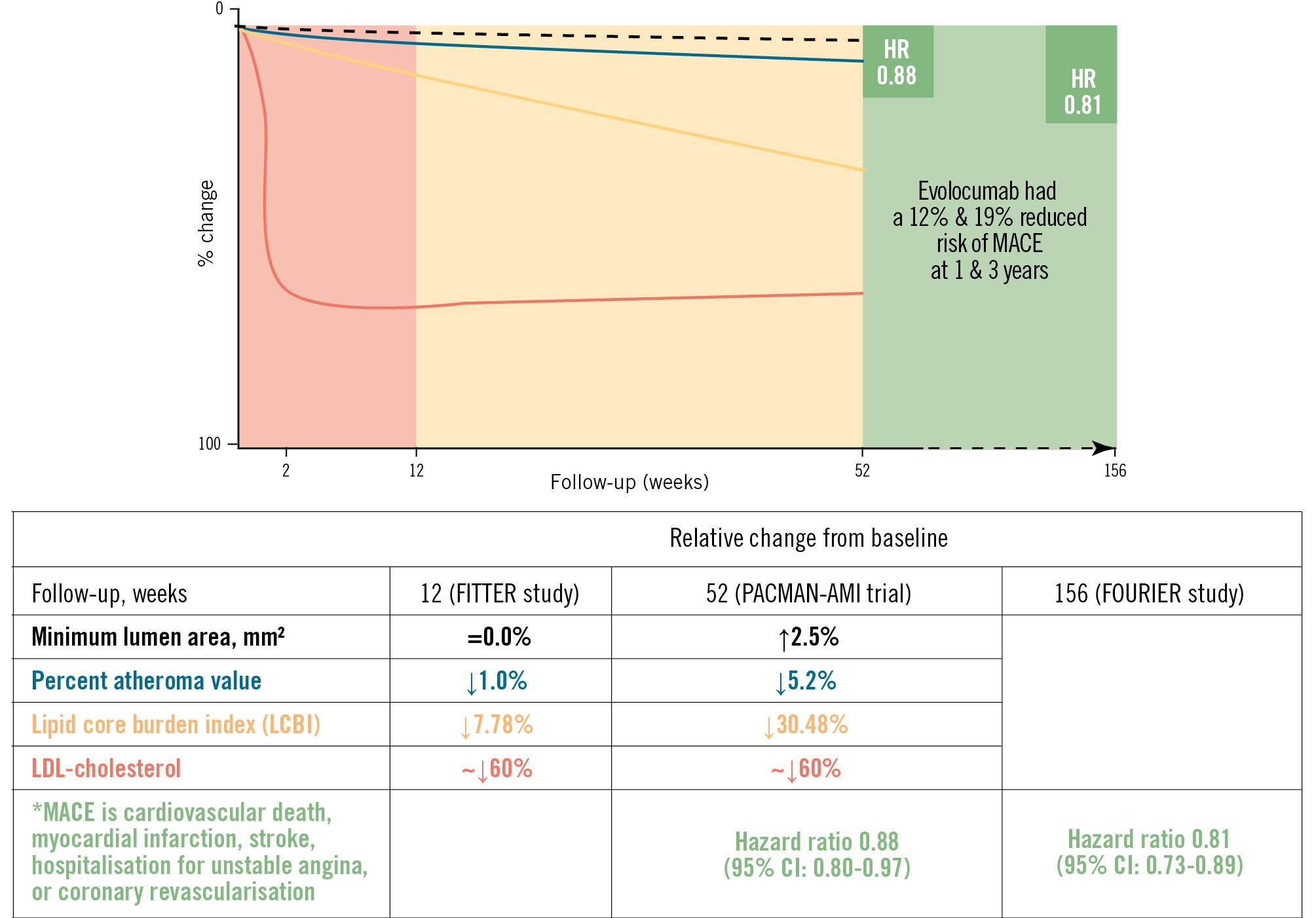
Figure 1. Proprotein convertase subtilisin/kexin type 9 inhibitors' effects on coronary plaque and outcomes. Blood (shaded red) and plaque (shaded orange) changes are observed before clinical outcomes (shaded green). The graph shows the overall rate of change in minimum lumen area (black dashed line), percent atheroma value (blue line), lipid core burden index (yellow line) and LDL-cholesterol (red line) in the FITTER study up to 12 weeks and in the PACMAN-AMI study up to 52 weeks. The far-right green shaded area shows the clinical outcome results up to 156 weeks in the FOURIER study. CI: confidence interval; HR: hazard ratio; LDL: low-density lipoprotein; MACE: major adverse cardiac event
Conflict of interest statement
H.M. Garcia-Garcia discloses institutional grants from Medtronic, Biotronik, Abbott, Neovasc, CorFlow, Philips, Chiesi, Boston Scientific, Cordis, Medis, Elixir, Terumo, and MedHub; consulting fees and honoraria from Boston Scientific, Medis, Abbott, and Terumo; and support for attending meetings from Boston Scientific, Abbott, and Terumo.

