Cory:
Unlock Your AI Assistant Now!
Contemporary drug-eluting stents (DES) have significantly reduced target lesion failure (TLF) rates after percutaneous coronary intervention (PCI), yet challenges remain. The dual-therapy sirolimus-eluting and CD34 antibody-coated COMBO stent (DTS; OrbusNeich) was designed to enhance early healing1. In the SORT OUT X trial, the sirolimus-eluting Orsiro stent (SES; Biotronik) was found to be superior to the DTS after 12 months, mainly because the DTS was associated with an increased risk of target lesion revascularisation (TLR)2. At 3 years, the SES was still superior to the DTS, mainly because the DTS was associated with an increased risk of target lesion failure within the first year but not from 1 to 3 years3. Lately, the DTS was found to be non-inferior to the biolimus A9-eluting BioMatrix Alpha stent (Biosensors International)4. In general, collecting long-term data is pivotal for understanding the behaviour of certain technologies over time. Furthermore, the DTS has been reported to show a unique late neointimal regression that has not been reported for any other DES5. The present study extends the SORT OUT X follow-up to 5 years.
The SORT OUT X trial is a prospective multicentre randomised clinical trial with registry-based follow-up2. Patients were randomised to receive DTS (n=1,578) or SES (n=1,568). The primary endpoint was TLF, a composite of cardiac death, myocardial infarction, and TLR.
At five years, 218 patients (13.8%) in the DTS group and 179 patients (11.4%) in the SES group met the primary endpoint (relative risk [RR] for TLF 1.24, 95% confidence interval [CI]: 1.01-1.51; p=0.04). The higher TLF rate in the DTS group within the first year was primarily due to increased TLR. From one to five years, TLF rates were similar between the groups (Figure 1). There were no differences in cardiac death (RR 1.07, 95% CI: 0.79-1.46; p=0.65), myocardial infarction (RR 1.09, 95% CI: 0.82-1.46; p=0.55) or stent thrombosis (RR 1.44, 95% CI: 0.76-2.73; p=0.26) at any point in time.
The findings from the SORT OUT X trial provide valuable insights into the comparative efficacy of DTS and SES in PCI. The higher TLF rate observed in the DTS group within the first year suggests that while the dual-therapy approach may enhance early endothelial healing, it may not translate into superior long-term outcomes compared to SES using the guideline-directed duration of dual antiplatelet therapy (DAPT).
One possible explanation for the increased TLR in the DTS group is the presence of the CD34 antibody coating, which is intended to promote endothelial progenitor cell capture and accelerate healing. However, this accelerated healing might lead to neointimal hyperplasia, contributing to higher rates of restenosis and subsequent revascularisation. This phenomenon underscores the complexity of stent design and the need for a balanced approach that optimises both early and long-term outcomes. As discussed in the previous SORT OUT X publications, differences in stent design including drug-eluting kinetics and stent strut thickness might contribute to the observed differences23. Whether or not the intended early healing with the CD34 antibody coating protects against subacute stent thrombosis in patients with a short DAPT duration was not evaluated in SORT OUT X.
Even though the similar TLF rates between DTS and SES from one to five years indicate that the initial disadvantages of DTS diminish over time, this study shows that the theoretical benefits of the DTS are undermined by the higher early revascularisation rates. Further studies are needed to conclude whether the DTS still holds niche value in specific patient subsets (e.g., high bleeding risk or endothelial dysfunction), but its broad clinical utility is limited compared to more established DES platforms.
The findings also have implications for future stent design and development. The dual-therapy approach requires further refinement to balance early healing with long-term efficacy. Innovations in stent technology, such as bioresorbable scaffolds or advanced drug coatings, could potentially address the limitations observed in the DTS group.
In conclusion, the SES demonstrated superior five-year outcomes compared to the DTS, mainly due to reduced TLF within the first year. Rates of all-cause death, cardiac death, and myocardial infarction at five years did not differ significantly between the two stent groups. Future studies should focus on refining dual-therapy stent designs to enhance both early and long-term efficacy.
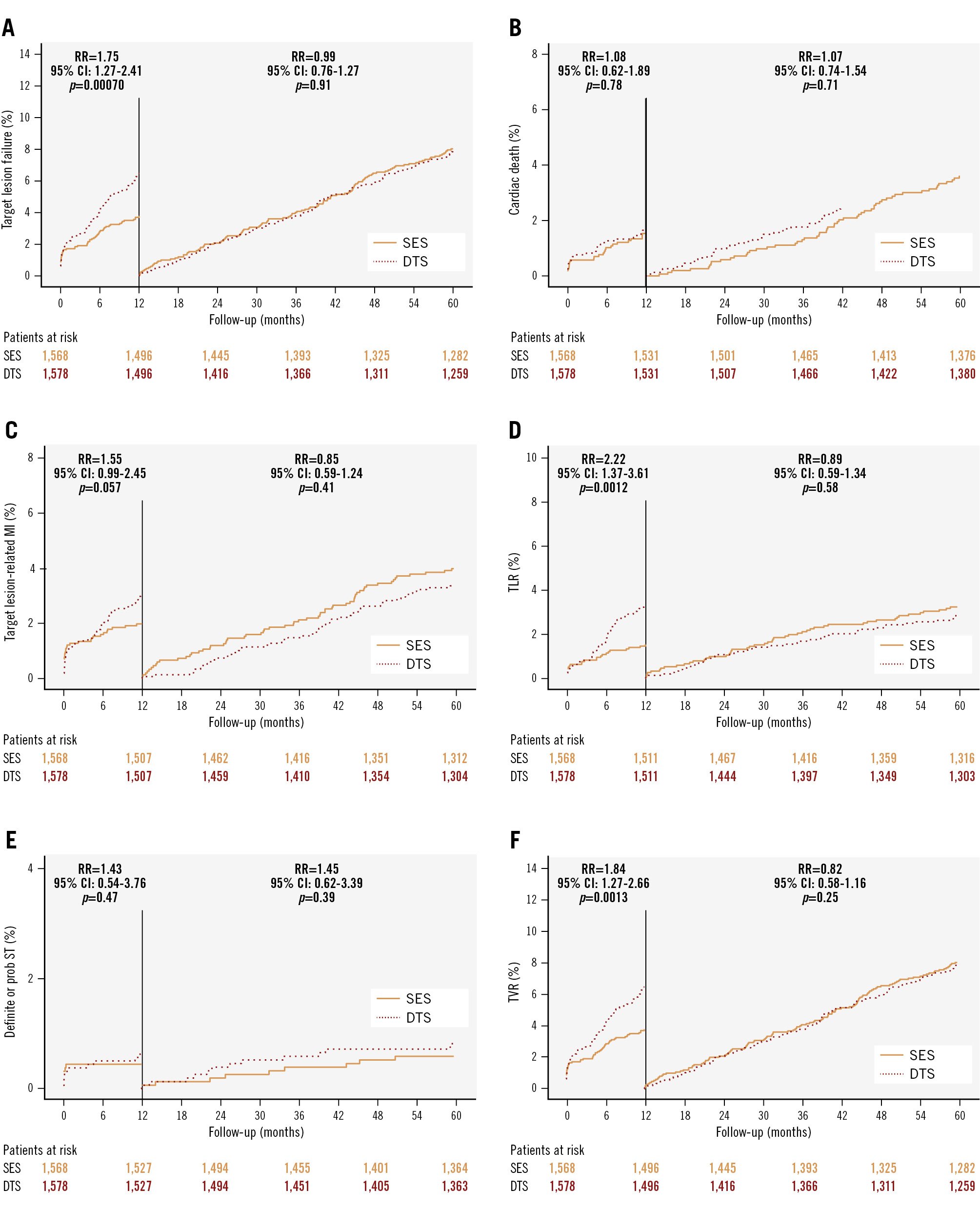
Figure 1. Time-to-event curves with landmark analyses for the primary and secondary outcomes. The relative risk from 0-1 year and from 1-5 years with 95% confidence intervals and p-values are also presented. A) Target lesion failure, (B) cardiac death, (C) target lesion-related myocardial infarction, (D) target lesion revascularisation, (E) definite or probable stent thrombosis, (F) target vessel revascularisation. CI: confidence interval; DTS: dual-therapy CD34 antibody-covered sirolimus-eluting stent; MI: myocardial infarction; RR: relative risk; SES: sirolimus-eluting stent; ST: stent thrombosis; TLR: target lesion revascularisation; TVR: target vessel revascularisation
Funding
The study is supported with equal unrestricted grants from Biotronik, Bülach, Switzerland, and OrbusNeich Medical, Fort Lauderdale, FL, USA. The sponsors had no role in designing the study, data collection, data analysis, interpretation of the data, writing the report, or the decision to submit the paper for publication. The corresponding author had full access to all the data in the study and had the final responsibility for the decision to submit the paper for publication.
Conflict of interest statement
L.O. Jensen has received research grants from Biotronik, OrbusNeich, Biosensors, and Terumo, to her institution; and honoraria from Biotronik. E.H. Christiansen has received grants from OrbusNeich and Biotronik, to his institution. The other authors have no conflicts of interest to declare.

