Cory:
Unlock Your AI Assistant Now!
The self-expanding ACURATE transcatheter heart valve (Boston Scientific) received European Conformity (CE) mark approval in 2011 and has since progressed to its third-generation iteration, from “neo” to “neo2” to “Prime” (Figure 1). Despite its successful use for over a decade, on 28 May 2025, Boston Scientific announced the discontinuation of the ACURATE platform. This decision is considered a consequential response to disappointing results in the randomised IDE trial with ACURATE neo2 (ClinicalTrials.gov: NCT03735667), which was preceded several years ago by negative outcomes in the SCOPE I (NCT03011346) and SCOPE II (NCT03192813) trials with the first-generation ACURATE neo valve (Figure 1). Hence, critical voices may perceive this withdrawal as scientific reckoning, but this warrants a nuanced interpretation. The SCOPE trials lacked computed tomography core laboratory adjudication and centralised screening and included sites with limited experience using this platform. The outcomes in the IDE trial were largely driven by underexpansion – a procedural issue that was found to be preventable and correctable when adhering to the manufacturer’s procedural recommendations, rather than being a device-inherent flaw1. Even though only hypothesis-generating, these insights have since contributed to the field’s perception of valve underexpansion that can occur with all transcatheter aortic valve implantation (TAVI) platforms and will help to reinforce procedural best practices. It is worth mentioning that in the IDE trial, only a mean of 3 ACURATE implantations were performed per centre and year, with approximately 75% of operators performing fewer than five cases over the study period of four years. The main issue in the IDE trial, however, may have been that the balloon sizes for pre- and post-dilatation frequently were smaller than recommended by the screening committee. In contrast to trial data, several large-scale European observational registries demonstrated non-inferior outcomes in experienced hands, suggesting that ACURATE’s clinical potential may have been underestimated in the trials23 – even though the inherently lower evidence level of observational studies should be taken into consideration. The platform offered several particularly advantageous features: intuitive deployment, haemodynamic stability during the deployment, reliable commissural alignment, preserved coronary access, minimal coronary obstruction risk, and low pacemaker rates. Furthermore, outcomes in the treatment of patients with pure aortic regurgitation, failed surgical valves, and redo-TAVI were promising4. These characteristics have become increasingly relevant in complex anatomical settings and for lifetime management considerations. As a matter of fact, the initial enthusiasm among competitors regarding increased market share may be tempered by the realisation that ACURATE had often been used in challenging anatomies – such as small annuli, horizontal aortas, short coronary heights, and tortuous vasculature − which may be more demanding when using alternative platforms. Unfortunately, the controversy surrounding the ACURATE platform will now remain unresolved. A definitive resolution would have required a robust trial design that ensured appropriate device use – incorporating core laboratory adjudication, proper sizing recommendations by an experienced screening committee, careful patient selection, and strict adherence to recommended procedural steps. This has been largely applied in the randomised DOUBLE-CHOICE trial (Clinical Trials. gov: NCT05036018) where the primary combined endpoint at 30 days occurred in 15.4% among ACURATE neo2 recipients versus 30.4% among patients receiving Evolut valves (95% confidence interval: 9.1 to 20.7; p<0.001), albeit this difference was mainly driven by pacemaker implantations, and the more relevant 1-year outcomes are pending5. Admittedly, these very prerequisites – including the lack of long-term data – reflect the inherent limitation of the ACURATE platform, which may have been less forgiving than other valve types. Whether this represents an intrinsic device-specific issue or is merely related to the early-phase learning curve – similar to what we experienced with other platforms in the initial era of TAVI – remains an open question. The imposed requirements by the notified body for an extension of the CE mark may have constituted the final death blow for ACURATE. According to Boston Scientific, a clinical follow-up of all European ACURATE recipients was demanded that was hardly viable and disproportionate, particularly in light of the risk-based principles outlined in the EU Medical Device Regulation. Have recent European data, including the 3-year results of SCOPE I – which showed similar outcomes, with event curves crossing at 3 years despite ACURATE failing to meet non-inferiority at 30 days – been ignored by the regulatory authorities6? Notably, the withdrawal of the ACURATE platform was driven by unfavourable outcomes associated with the earlier generation used in the IDE trial, the ACURATE neo2, without clear evidence that these limitations extend to the latest Prime iteration, which features enhanced radial force with a rapid and stable deployment and actually has demonstrated favourable results in a multicentre registry (Ruck, A. Early ACURATE prime: multicenter study to evaluate safety and effectiveness. EuroPCR 2025. 20-23 May 2025. Paris, France). While we can understand the decision of Boston Scientific leadership to discontinue ACURATE from a strategic perspective, we as implanting physicians are convinced that this platform would have deserved a further chance – with a bit more perseverance, involvement of expert operators, and evaluation of more recent data that are expected soon in the decision process. Most patients will remain well served by alternative devices. For some interventionalists, the discontinuation of ACURATE represents a meaningful loss, potentially limiting the ability to tailor valve selection in certain anatomical scenarios within contemporary TAVI practice. 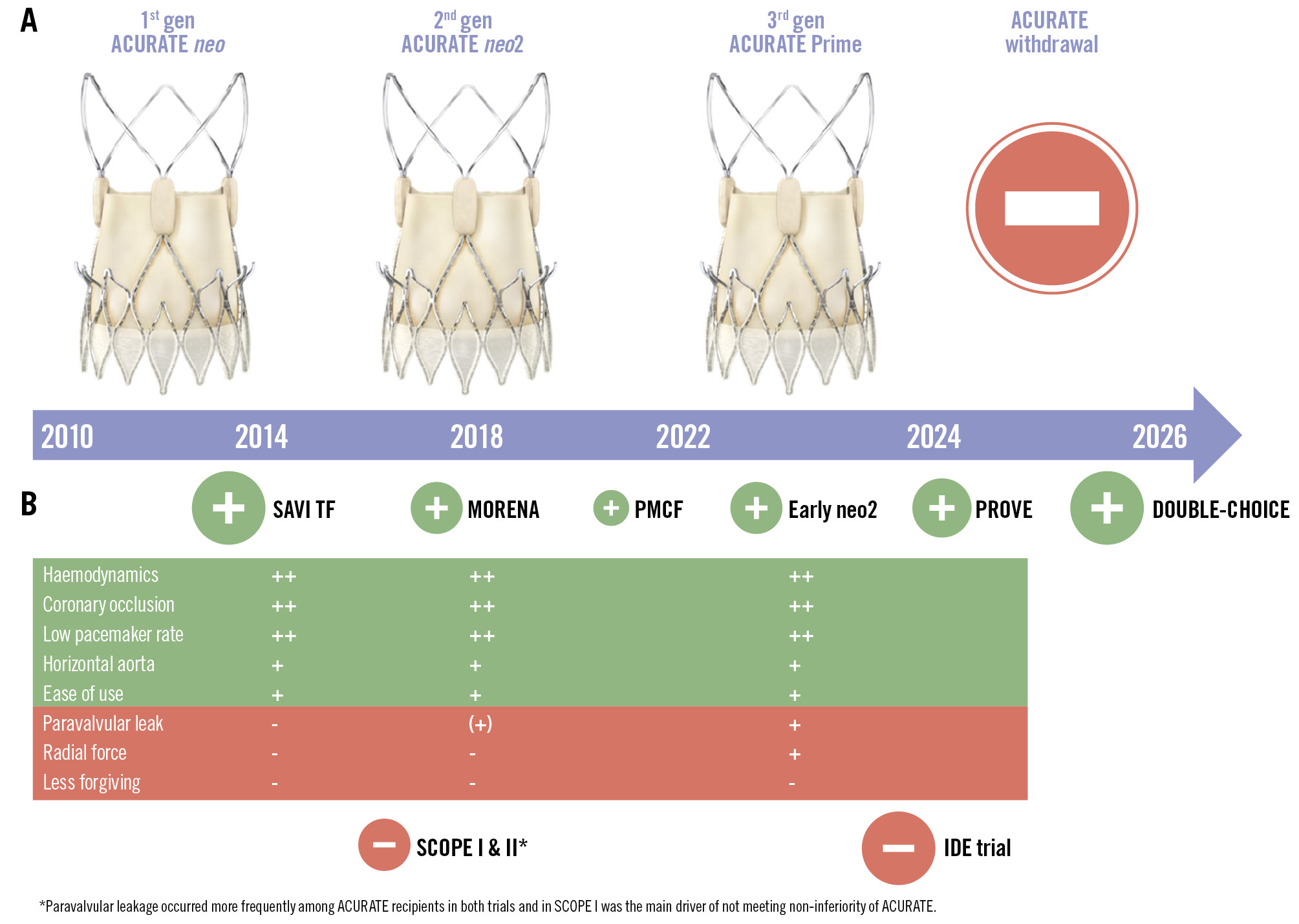
Figure 1. Overview of the ACURATE platform. Timeline and iterations of the ACURATE transcatheter heart valve platform are shown in (A). The neo2 featured an extended sealing skirt, whereas the Prime was characterised by enhanced radial force and streamlined deployment. B) Available evidence, in circles sized according to the study size, with favourable studies in green and unfavourable studies in red. The table summarises strengths (highlighted in green) and weaknesses (highlighted in red) of the different iterations. -: unfavourable; (+): acceptable; +: good; ++: excellent; DOUBLE-CHOICE: ClinicalTrials.gov: NCT05036018; Early neo2 Registry, NCT04810195; MORENA: Multicenter Comparison of Novel Self-Expanding versus Balloon-Expandable Transcatheter Heart Valves; PMCF: Post Market Clinical Follow up Study, NCT04655248; PROVE: NCT05539573; SAVI TF: NCT02306226; SCOPE I: NCT03011346; SCOPE II: NCT03192813
Acknowledgements
The authors wish to thank Holger Thiele, MD, Mohamed Abdel-Wahab, MD, and Thomas Walther, MD, for their valuable input and thoughtful discussions that contributed to the development of this Viewpoint.
Conflict of interest statement
W.-K. Kim: proctor/speaker honoraria from Abbott, Anteris, Boston Scientific, Edwards Lifesciences, JenaValve, Hi-D Imaging, Meril Life Sciences, and P&F; and institutional fees from Boston Scientific. H. Möllmann: proctor/speaker honoraria from Abbott, Boston Scientific, and Edwards Lifesciences.

