Cory:
Unlock Your AI Assistant Now!
The role of beta blockers after myocardial infarction (MI) in the reperfusion era has been challenged by four major contemporary randomised controlled trials: REDUCE-AMI1, AβYSS2, REBOOT3 and BETAMI-DANBLOCK4, which together with the older CAPITAL-RCT5 enrolled more than 23,000 patients across 10 countries. This represents a major accomplishment for the academic community. Results, however, remain heterogeneous. CAPITAL-RCT (801 patients) was stopped prematurely and showed only a non-significant trend towards a benefit of carvedilol. REDUCE-AMI (5,020 patients) and REBOOT (8,438 patients) both reported neutral results, with no difference in death or MI. In contrast, BETAMI-DANBLOCK (5,574 patients) found a significant reduction in a larger composite outcome of death from any cause, MI, unplanned coronary revascularisation, ischaemic stroke, heart failure, or ventricular arrhythmias with continued beta blocker therapy, while AβYSS (3,698 patients), which specifically tested withdrawal after chronic use, showed more reinfarctions and hospitalisations in the withdrawal arm. These divergences have fuelled ongoing debate between advocates and sceptics.
The first consistent finding across trials is that beta blockers at contemporary doses are well tolerated. While historically considered poorly tolerated, this perception has been refuted in recent studies. Neither initiation (REDUCE-AMI) nor withdrawal (AβYSS) had a measurable impact on quality of life using EuroQol 5-Dimension 5-Level (EQ-5D-5L) questionnaires, challenging the idea that discontinuation is justified by intolerance16. A second area of consistency concerns left ventricular function. The recent individual patient-data meta-analysis on a selected subgroup of 1,885 patients with mildly reduced ejection fraction (40-50%)7 confirmed a consistent prognostic benefit. This reinforces the long-recognised gradient of effect, where infarct size and residual myocardial damage drive both risk and treatment effect.
The global interpretation of these trials remains challenging, as their heterogeneity mainly stems from differences in endpoint selection, with some focused on strict hard outcomes and others incorporating softer criteria such as hospitalisations. At first glance, it almost appears that trials with hard endpoints tended to be neutral, while those using broader composites were positive, largely driven by rehospitalisations and recurrent MI. This underscores the challenge of statistical power in both global and subgroup analyses.
To add to the debate, we performed a pooled analysis of all five trials for this editorial, without restricting the endpoint, population, or phase of beta blocker use. Among 23,598 patients, beta blocker therapy was associated with a pooled hazard ratio (HR) of 0.91 (95% confidence interval [CI]: 0.84-0.98; p=0.009 for fixed effects; 95% CI: 0.83-0.99; p=0.035 for random effects) (Figure 1). Heterogeneity was low (I²=26%; p=0.25), supporting consistency of the effect despite differences in design. Together, REBOOT and AβYSS represent 63% of the event weight of this pooled analysis, and restricting the analysis to hard endpoints (such as death, MI, or heart failure) eliminated the modest observed benefit. Exclusion of AβYSS, which was performed in the chronic phase, attenuated the benefit (HR 0.93, 95% CI: 0.82-1.04) and increased heterogeneity. The same analysis suggested a borderline reduction in recurrent MI (HR 0.89; p=0.07) and a highly homogeneous signal for fewer heart failure hospitalisations (HR 0.81; p=0.13). This global analysis raises two central questions: should the interpretation of these beta blocker trials rely on softer endpoints that capture morbidity and hospitalisations or be limited to harder outcomes; and should the evidence from acute-phase and chronic-withdrawal trials be considered together?
While awaiting more evidence from ongoing randomised trials performed in the chronic phase, such as SMART-DECISION8 and ABBREVIATE (ClinicalTrials.gov: NCT05081999), Rossello and colleagues provide important insights in this issue of EuroIntervention through a post hoc analysis of REBOOT9. They demonstrated that beta blocker withdrawal did not provoke a short-term rebound risk and its overall effect remained neutral at almost 4 years, independent of prior exposure. However, recurrent infarctions were numerically more frequent in the withdrawal arm − a signal consistent with BETAMI-DANBLOCK and AβYSS − raising concern that longer-term discontinuation may still carry risk in selected populations.
Altogether, the evidence shows that beta blockers remain most valuable in MI patients with a reduced or mildly reduced ejection fraction, larger infarcts, incomplete revascularisation, arrhythmic substrate, or recurrent events. For carefully selected low-risk patients, discontinuation may be safe regarding hard outcomes, but it provides no quality-of-life benefit, raises concerns about high blood pressure control10, and removes a modest signal for morbidity reduction. Thus, beta blocker withdrawal may be safe for the few, but continuation remains relevant for the many.
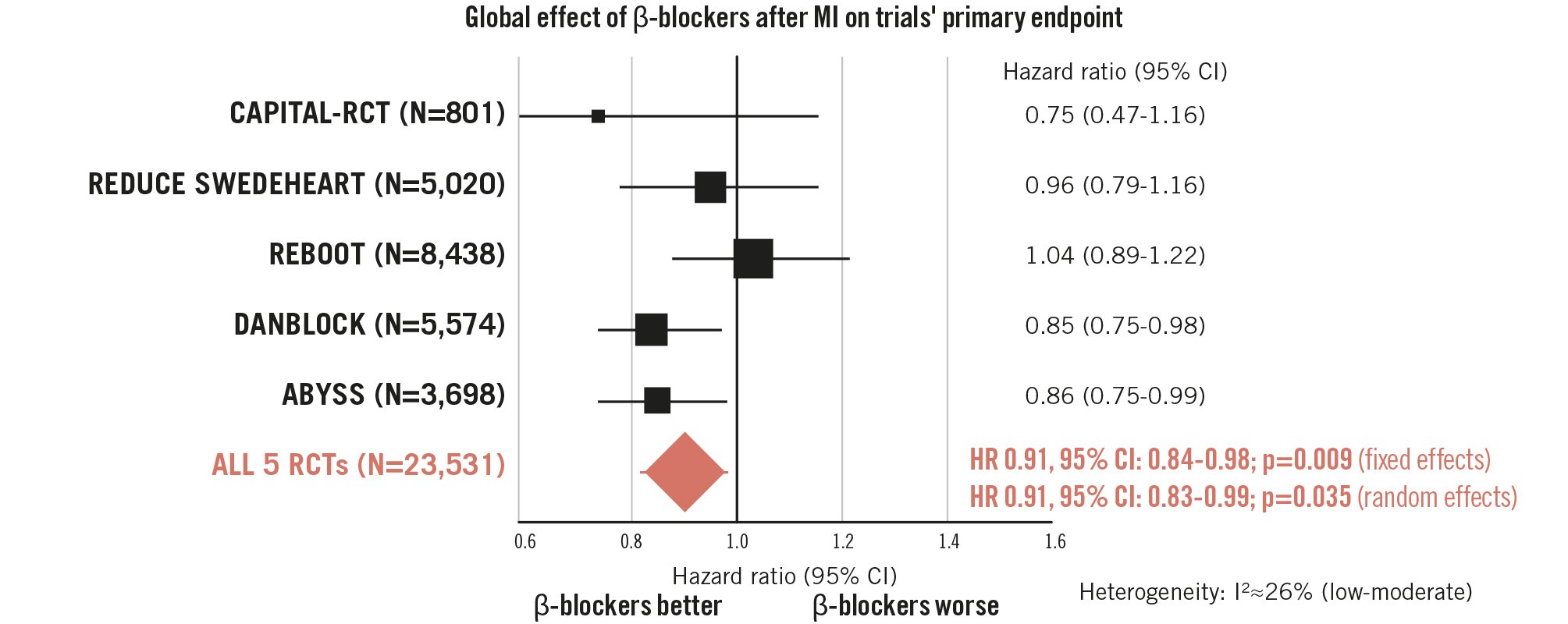
Figure 1. Pooled meta-analysis of 23,531 post-MI patients, evaluating the effect of beta blockers in the modern era using each trial’s primary endpoint and overall population. CI: confidence interval; HR: hazard ratio; MI: myocardial infarction; RCT: randomised controlled trial
Conflict of interest statement
J. Silvain declares the following relation with the industry during the last 2 years: payment or honoraria for lectures, presentations, speakers bureau, manuscript writing or educational events: Abbott and SMT; consulting fees or lecture fees: Biotronik, Sanofi Aventis, and CSL Behring; travel support, hospitality: Abbott, Biotronik, Medtronic, and Novo Nordisk; stockholder: 4P-Pharma; participation on a data safety monitoring board or advisory board: Amgen. N. Procopi has no conflicts of interest to declare.

