Cory:
Unlock Your AI Assistant Now!
Abstract
Background: The new-generation supra-annular, self-expanding Evolut FX system has the potential to facilitate commissural alignment.
Aims: We sought to assess the feasibility of coronary cannulation (CC) and the impact of commissural and coronary alignment on CC execution, as confirmed by post-transcatheter aortic valve implantation (TAVI) computed tomography (CT).
Methods: The CANNULATE TAVR EXPANDED study is a multicentre, prospective study which included consecutive patients who underwent transfemoral TAVI with the Evolut FX, CC, and angiography after valve deployment. Post-TAVI CT was performed to assess commissural and coronary alignment. Moderate-to-severe commissural and coronary misalignments based on the ALIGN-TAVR Consortium definition were categorised as the misalignment group. The primary endpoint was the rate of successful CC after Evolut FX implantation.
Results: A total of 126 patients were included. CC was successful in 100% of cases for the left coronary artery (LCA) and 96.7% for the right coronary artery (RCA). Moderate-to-severe commissural misalignment was observed in 13.5%, and moderate-to-severe coronary misalignment was observed in 20.6% (LCA) and 22.2% (RCA). Misaligned LCA and RCA required significantly longer CC times. In multivariable analysis, factors associated with suboptimal LCA cannulation were coronary height (odds ratio [OR] 0.73, 95% confidence interval [CI]: 0.57-0.90; p=0.006) and coronary misalignment (OR 4.58, 95% CI: 1.45-14.47; p=0.009), whereas right coronary cusp width (OR 0.63, 95% CI: 0.44-0.90; p=0.007) and coronary misalignment (OR 4.64, 95% CI: 1.29-16.74; p=0.019) were identified for the RCA.
Conclusions: High rates of CC, and commissural and coronary alignment post-TAVI with the Evolut FX were observed in this prospective, multicentre study. Coronary misalignment was identified as the strongest predictor of suboptimal CC for both the LCA and the RCA.
As transcatheter aortic valve implantation (TAVI) expands to younger, lower-risk patients with longer life expectancies, lifetime management including the need for future coronary cannulation (CC) becomes crucial. While challenges in CC after TAVI with tall-frame, supra-annular as compared to short-frame, intra-annular transcatheter heart valves (THVs) have been reported, those studies were hindered by the absence of commissural alignment enforcement during the procedure1 and by the absence of post-TAVI computed tomography (CT) to better determine both commissural and coronary alignment12. In order to optimise commissural alignment following TAVI, device-specific methods have been proposed and have proven to be helpful3. The new iteration of the supra-annular, self-expanding Evolut FX system (Medtronic), whose 3 markers are designed to align with the valve commissures, has the potential to facilitate commissural alignment4. We therefore aimed to prospectively assess the impact of commissural and coronary alignment, as determined by postprocedural CT, on the feasibility and timing of CC after TAVI with the Evolut FX in consecutive patients.
Methods
Study population and design
CANNULATE TAVR EXPANDED is an investigator-driven, prospective, multicentre study enrolling consecutive patients undergoing TAVI for severe symptomatic aortic stenosis (AS) using the Evolut FX and subsequent CC and angiography immediately after valve implantation from March 2023 to February 2024. Patients undergoing a valve-in-valve procedure, those undergoing non-transfemoral access, those having an Evolut FX that was not implanted into the proper anatomical position, and those with unstable haemodynamics were excluded. All patients underwent multidetector CT before and after TAVI. The study protocol was developed in accordance with the Declaration of Helsinki and was approved by the ethics committee of each participating hospital. All patients provided informed consent prior to participating in this prospective registry.
Periprocedural CT imaging
Pre-TAVI analysis
Aortic root complex measurement was performed as previously described5. Coronary heights were measured from the annular plane to the inferior border of each coronary ostium in a stretched multiplanar image. Sinus of Valsalva (SoV) diameters were tracked from the commissure to the opposite side of each coronary sinus. The sinotubular junction (STJ) width was measured as an average of the shortest and longest diameters at the level of the STJ. The angles between the commissure and each coronary ostium were measured. The percentage of annular oversizing was calculated as (THV perimeter/annular perimeter – 1) × 100. The THV-SoV relation percentage was calculated as (THV diameter/SoV mean diameter – 1) × 1001.
Post-TAVI analysis
Using the 3 orthogonal multiplanar reconstruction planes, a centreline perpendicular to the THV was generated, and the outflow and inflow levels of each THV were identified. The angle between the native commissure and the THV commissure was assessed using end-diastolic phase data. At the cross-sectional level of the THV leaflet coaptation, the positions of the THV commissures were identified and marked. Then, the native commissures were identified in a cross-section perpendicular to the axis of the aorta, and the angle relative to the centre of the THV frame was measured. Likewise, the ostium of each coronary was identified in a similar fashion, and 2 angles were measured: from the left coronary artery (LCA) ostium to its nearest THV commissure marker, and from the right coronary artery (RCA) ostium to its nearest THV commissure marker6. The horizontal distance between the THV and STJ was measured. The definitions for the degrees of commissural and coronary misalignments were based on the Alignment of Transcatheter Aortic-Valve Neo-Commissures (ALIGN-TAVR) Consortium7. Moderate-to-severe commissural and coronary misalignment were categorised as the misaligned group, and two groups (aligned vs misaligned) were compared for further analysis. CT analysis post-TAVI was performed by experienced analysts with documented high reproducibility6.
Procedure details
All enrolled patients underwent transfemoral TAVI using the Medtronic Evolut FX system. Prosthesis size and access site were decided by the local Heart Team based on the findings of preprocedural echocardiography and multidetector CT images. Detailed TAVI procedures have been previously described8. All the procedures followed the current best practice (Figure 1): an Evolut FX was inserted with the flush port of the delivery catheter positioned at 3 o’clock, and the position of the hat marker was assessed at the descending aorta. If the hat marker was not located at the outer curve of the descending aorta in the left anterior oblique (LAO) projection, the delivery system was rotated counterclockwise to achieve the optimal orientation (Figure 1A). The position of the hat marker was again assessed at the ascending aorta, to determine whether it remained at the outer curve (Figure 1B). Finally, valve orientation was confirmed in the cusp-overlap view and implanted in the same projection (Figure 1C). Commissural alignment on fluoroscopy was defined as 1 marker at the left-right commissure (right side) and 2 markers towards the non-coronary cusp (left side) of the annulus in the cusp-overlap view (Figure 1D). Immediately after valve implantation, CC of both the LCA and RCA was attempted via either a femoral or radial approach. Judkins left (JL) 3.5 and Judkins right 4 diagnostic catheters were used as default catheters to engage the LCA and RCA, but different types of diagnostic catheters were chosen per the operators’ discretion if the initial catheters were deemed inadequate9. Coronary guidewires or guide extension catheters were not allowed to be used for coronary cannulation.
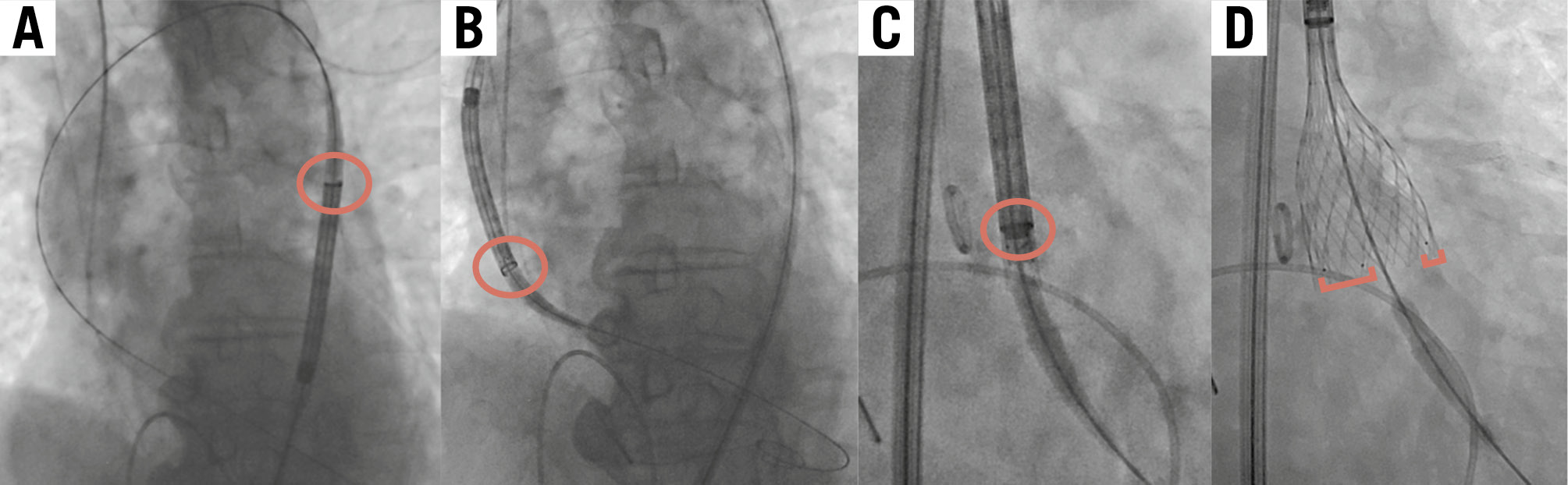
Figure 1. Steps of transfemoral TAVI using the Evolut FX. This figure shows the key steps of transfemoral TAVI using the cusp-overlap technique. The red circles show the hat marker; the red brackets show the golden markers. A) The hat marker faces the outer curve of the descending aorta in the LAO projection. B) The hat marker faces the outer curve of the ascending aorta in the LAO projection. C) The hat marker faces “centre front” in the cusp-overlap view. D) One marker is at the left-right commissure (right side) and two markers are towards the non-coronary cusp (left side) of the annulus in the cusp-overlap view. LAO: left anterior oblique; TAVI: transcatheter aortic valve implantation
Endpoints and definitions
The primary endpoint was the rate of successful CC after Evolut FX implantation. Secondary endpoints were the identification of factors associated with the failed or delayed CC. Consistently with prior studies, CC was defined as “selective” when the catheter completely engaged the coronary ostium; “non-selective” when the catheter tip was near the coronary ostium without complete engagement, but resulting in adequate opacification of the coronary; or “failed” when it was deemed impossible to obtain selective or non-selective coronary engagement, thus precluding proper opacification of the coronary artery with contrast media injection12. CC was deemed “successful” when the demonstration of opacification of the coronary artery with the proximity of the catheter tip to the coronary ostium, including non-selective injections, was possible within 10 minutes. We defined suboptimal CC as cases of “failed” or longer cannulation time (longer than the third quartile of cannulation time) with non-selective cannulation. All clinical and echocardiographic outcomes were defined according to the Valve Academic Research Consortium-3 criteria10.
Statistical analysis
Continuous variables are expressed as mean±standard deviation or median (interquartile range [IQR]), as appropriate. Categorical variables are described as frequency and percentage. Continuous variables were compared using the Student’s t-test or the Mann-Whitney U test depending on the variable’s distribution, whereas an analysis of variance (ANOVA) test or the Kruskal-Wallis test were used for comparing more than 2 groups for normally distributed and skewed variables, respectively. Categorical variables were compared by the chi-square or Fisher’s exact test. Factors associated with failed/delayed CC were assessed using logistic regression analyses. All variables that were significantly associated with the outcome of interest at univariate analysis (p<0.10) and those considered clinically relevant were then included in the multivariable analysis. Results are reported as odds ratios (ORs) with 95% confidence intervals (CIs). All p-values reported are 2-sided, and p values<0.05 were considered significant. All analyses were performed with JMP, version 15 (SAS Institute).
Results
Baseline and procedural characteristics
A total of 126 patients (45% female) with a median age of 81 years were enrolled in the CANNULATE TAVR EXPANDED study. Baseline demographic, clinical, and CT characteristics according to commissural alignment are summarised in Table 1. Baseline CT data including annular size (perimeter and area), coronary heights, sinus of Valsalva width, STJ width, angles between the commissure and each coronary, and aortic root angles were comparable between the 2 groups. All the TAVI procedures followed the current best practice, and no cases required a delivery catheter being retrieved from the ascending aorta to the descending aorta after crossing the aortic arch. The sizes of Evolut FX used in this study were also similar between the groups. The median implantation depth was 3 (IQR 3-3) mm at the non-coronary cusp (NCC) and 4 (IQR 3-6) mm at the left coronary cusp (LCC) (Table 2).
Table 1. Baseline patient characteristics.
| Commissural alignment | |||||
|---|---|---|---|---|---|
| Baseline characteristics | N | Overall | Aligned | Misaligned | p-value |
| N | 126 | 126 | 109 | 17 | |
| Age, yrs | 126 | 81757677787980818283848586 | 81757677787980818283848586 | 817677787980818283848586 | 0.75 |
| Female | 126 | 57 (45) | 48 (44) | 9 (53) | 0.49 |
| Past medical history | |||||
| Hypertension | 126 | 111 (88) | 94 (86) | 17 (100) | 0.10 |
| Dyslipidaemia | 126 | 101 (80) | 88 (80) | 13 (76) | 0.68 |
| Diabetes | 126 | 45 (36) | 39 (36) | 6 (35) | 0.97 |
| Coronary artery disease | 126 | 45 (36) | 42 (39) | 3 (18) | 0.09 |
| CABG | 126 | 12 (10) | 12 (11) | 0 (0) | 0.15 |
| Baseline CT data | |||||
| Annular perimeter, mm | 126 | 76.3 [71.2-82.1] | 76.7 [71.3-82.4] | 74.8 [67.1-81.2] | 0.25 |
| Annular area, mm2 | 126 | 432384385386387388389390391392393394395396397398399400401402403404405406407408409410411412413414415416417418419420421422423424425426427428429430431432433434435436437438439440441442443444445446447448449450451452453454455456457458459460461462463464465466467468469470471472473474475476477478479480481482483484485486487488489490491492493494495496497498499500501502 | 436387388389390391392393394395396397398399400401402403404405406407408409410411412413414415416417418419420421422423424425426427428429430431432433434435436437438439440441442443444445446447448449450451452453454455456457458459460461462463464465466467468469470471472473474475476477478479480481482483484485486487488489490491492493494495496497498499500501502503504 | 419349350351352353354355356357358359360361362363364365366367368369370371372373374375376377378379380381382383384385386387388389390391392393394395396397398399400401402403404405406407408409410411412413414415416417418419420421422423424425426427428429430431432433434435436437438439440441442443444445446447448449450451452453454455456457458459460461462463464465466467468469470471472473474475476477478479480481482483484485486487488489490491492493494495496497 | 0.29 |
| LCA height, mm | 126 | 141213141516 | 141213141516 | 131213141516 | 0.48 |
| RCA height, mm | 126 | 16141516171819 | 16141516171819 | 151112131415161718 | 0.10 |
| LCC width, mm | 126 | 31293031323334 | 3129303132333435 | 31282930313233 | 0.17 |
| RCC width, mm | 126 | 30282930313233 | 31282930313233 | 292728293031 | 0.09 |
| STJ width, mm | 126 | 2825262728293031 | 2825262728293031 | 27232425262728293031 | 0.29 |
| LCA-comm angle, degrees | 126 | 645556575859606162636465666768697071 | 645556575859606162636465666768697071 | 67565758596061626364656667686970717273747576 | 0.44 |
| RCA-comm angle, degrees | 126 | 77697071727374757677787980818283848586 | 776869707172737475767778798081828384 | 85767778798081828384858687888990 | 0.05 |
| Aortic root angle, degrees | 126 | 50.0±9.4 | 49.7±9.1 | 51.8±11.0 | 0.38 |
| Bicuspid aortic valve | 126 | 6 (4.8) | 4 (3.7) | 2 (12) | 0.19 |
| Values are n (%), mean±standard deviation, or median [interquartile range]. CABG: coronary artery bypass graft; comm: commissure; CT: computed tomography; LCA: left coronary artery; LCC: left coronary cusp; RCA: right coronary artery; RCC: right coronary cusp; STJ: sinotubular junction | |||||
Table 2. Procedural characteristics based on commissural alignment.
| Commissural alignment | |||||
|---|---|---|---|---|---|
| Procedural characteristics | N | Overall | Aligned | Misaligned | p-value |
| N | 126 | 126 | 109 | 17 | |
| Evolut FX size | 126 | 0.30 | |||
| 23 mm | 15 | 11 (10) | 4 (24) | ||
| 26 mm | 31 | 27 (25) | 4 (24) | ||
| 29 mm | 49 | 42 (39) | 7 (41) | ||
| 34 mm | 31 | 29 (27) | 2 (12) | ||
| NCC depth, mm | 126 | 3 [3-3] | 3 [3-3] | 3 [3-5] | 0.33 |
| LCC depth, mm | 126 | 4 [3-6] | 4 [3-6] | 4 [4-6] | 0.85 |
| Valve angle, degrees | 126 | 42.6±8.8 | 42.3±8.9 | 44.6±8.4 | 0.33 |
| THV/annular oversizing by perimeter, % | 126 | 17.2 [13.1-21.4] | 17.3 [13.4-21.4] | 14.3 [12.1-20.6] | 0.26 |
| THV/SoV relation*, % | 126 | –7.5 [–13.4 to –3.2] | –7.2 [–13.4 to –3.0] | –8 [–14.8 to –3.3] | 0.74 |
| THV/SoV relation**, % | 126 | –26.5 [–31.3 to –23.0] | –26.7 [–31.4 to –23.3] | –24.5 [–28.7 to –22.0] | 0.23 |
| LCA cannulation | 126 | 126 | 109 | 17 | 0.36 |
| Selective | 93 | 82 (75) | 11 (65) | ||
| Non-selective | 33 | 27 (25) | 6 (35) | ||
| Failure | 0 | 0 | 0 | ||
| LCA cannulation time, sec | 126 | 99 [48-175] | 86 [47-165] | 188 [112-302] | 0.002 |
| RCA cannulation | 122 | 122 | 106 | 16 | 0.003 |
| Selective | 77 | 71 (67) | 6 (38) | ||
| Non-selective | 41 | 34 (32) | 7 (44) | ||
| Failure | 4 | 1 (1) | 3 (19) | ||
| RCA cannulation time, sec | 122 | 140 [70-290] | 140 [72-271] | 176 [60-592] | 0.34 |
| Coronary cannulation access | 126 | 126 | 109 | 17 | 0.24 |
| Via femoral | 95 | 80 | 15 | ||
| Via radial | 31 | 29 | 2 | ||
| Neo-comm-comm angle, degrees | 126 | 13 [6-22] | 10 [5-17] | 41 [39-45] | <0.001 |
| Commissural misalignment | 126 | 126 | 109 | 17 | <0.001 |
| None | 78 | 78 (72) | 0 | ||
| Mild | 31 | 31 (28) | 0 | ||
| Moderate | 13 | 0 | 13 (76) | ||
| Severe | 4 | 0 | 4 (24) | ||
| Neo-comm-LCA angle, degrees | 126 | 46 [34-53] | 49 [36-54] | 24 [15-37] | <0.001 |
| Coronary misalignment (LCA) | 126 | 126 | 109 | 17 | <0.001 |
| None | 64 | 62 (57) | 2 (12) | ||
| Mild | 36 | 31 (28) | 5 (29) | ||
| Moderate | 21 | 15 (14) | 6 (35) | ||
| Severe | 5 | 1 (1) | 4 (24) | ||
| Neo-comm-RCA angle, degrees | 126 | 44 [34-52] | 46 [36-53] | 25 [7-32] | <0.001 |
| Coronary misalignment (RCA) | 126 | 126 | 109 | 17 | <0.001 |
| None | 61 | 58 (53) | 3 (18) | ||
| Mild | 37 | 36 (33) | 1 (6) | ||
| Moderate | 20 | 12 (11) | 8 (47) | ||
| Severe | 8 | 3 (3) | 5 (29) | ||
| STJ-THV distance, mm | 126 | 2.5 [1.9-3.6] | 2.5 [2-3.7] | 2.7 [1.7-3.7] | 0.83 |
| Values are n, n (%), mean±standard deviation, or median [interquartile range]. *Calculated using the label size diameter provided by the THV manufacturer. **Calculated using the waist diameter provided by the THV manufacturer. comm: commissure; LCA: left coronary artery; LCC: left coronary cusp; NCC: non-coronary cusp; RCA: right coronary artery; SoV: sinus of Valsalva; STJ: sinotubular junction; THV: transcatheter heart valve | |||||
Commissural and coronary alignment
Post-TAVI CT was performed in all patients (126/126). Among them, moderate or severe commissural misalignment was observed in 13.5% (17/126) patients, and moderate or severe coronary misalignment was observed in 20.6% (26/126) and 22.2% (28/126) patients for the LCA and RCA, respectively (Central illustration). There was no significant difference regarding valve performance, i.e., mean pressure gradient and effective orifice area, between patients with commissural alignment and misalignment (mean pressure gradient 7.9 [IQR 5.8-10.5] mmHg vs 10.5 [IQR 7.4-13.7] mmHg; p=0.06, effective orifice area 1.7 [IQR 1.6-1.9] cm2 vs 1.7 [IQR 1.6-1.8] cm2; p=0.73, for commissural alignment vs misalignment, respectively). No patient with transvalvular aortic regurgitation was identified. Among the 17 patients with commissural misalignment, coronary misalignment was observed in 10 (58.8%) for the LCA and 13 (76.5%) for the RCA. Among the 109 patients with commissural alignment, coronary alignment was achieved in 93 (85.3%) for the LCA and 94 (86.2%) for the RCA.

Central illustration. Highlights of the CANNULATE TAVR EXPANDED study. This figure shows the highlights of the CANNULATE TAVR EXPANDED study. Coronary cannulation after TAVI with the Evolut FX was highly feasible due to high achievement rates of commissural and coronary alignment (A-C). Significantly longer cannulation times were required both for misaligned left and right coronary arteries (D). Coronary misalignment was strongly associated with suboptimal CC for both the LCA and the RCA (E). CC: coronary cannulation; CT: computed tomography; LCA: left coronary artery; RCA: right coronary artery; SoV: sinus of Valsalva; TAVI: transcatheter aortic valve implantation; TF: transfemoral
Coronary cannulation outcomes
Coronary artery cannulation was performed via the femoral artery in 75.4% of cases (95/126), while in 24.6% (31/126), it was performed via the radial artery. Coronary cannulation was successful in 100% (126/126) for the LCA and 96.7% (118/122) for the RCA. Selective cannulation was carried out in 73.8% (93/126) for the LCA and in 63.1% (77/122) for the RCA. The median times required for LCA and RCA cannulation were 99 [IQR 48-175] sec and 140 [IQR 70-290] sec, respectively. Four cases required switching the catheter from JL3.5 to JL4; among them, 2 cases were cannulated selectively, 2 cases non-selectively. No cases required an access site change for coronary cannulation. As for the approach site (femoral vs radial) of CC, when we combine the results of the left radial and femoral approaches due to the similarity of catheter trajectory, LCA cannulation time was comparable between the two approaches (p=0.56), whereas RCA cannulation time via the left radial/femoral artery was significantly shorter compared with the time via the right radial approach (p=0.006) (Supplementary Table 1, Figure 2A). The incidence of selective/non-selective cannulation for the LCA was comparable between the right radial versus the left radial approach. However, as for RCA cannulation, right radial approach cases had less frequent selective cannulation compared with the left radial approach, although it was not statistically significant between the two groups.
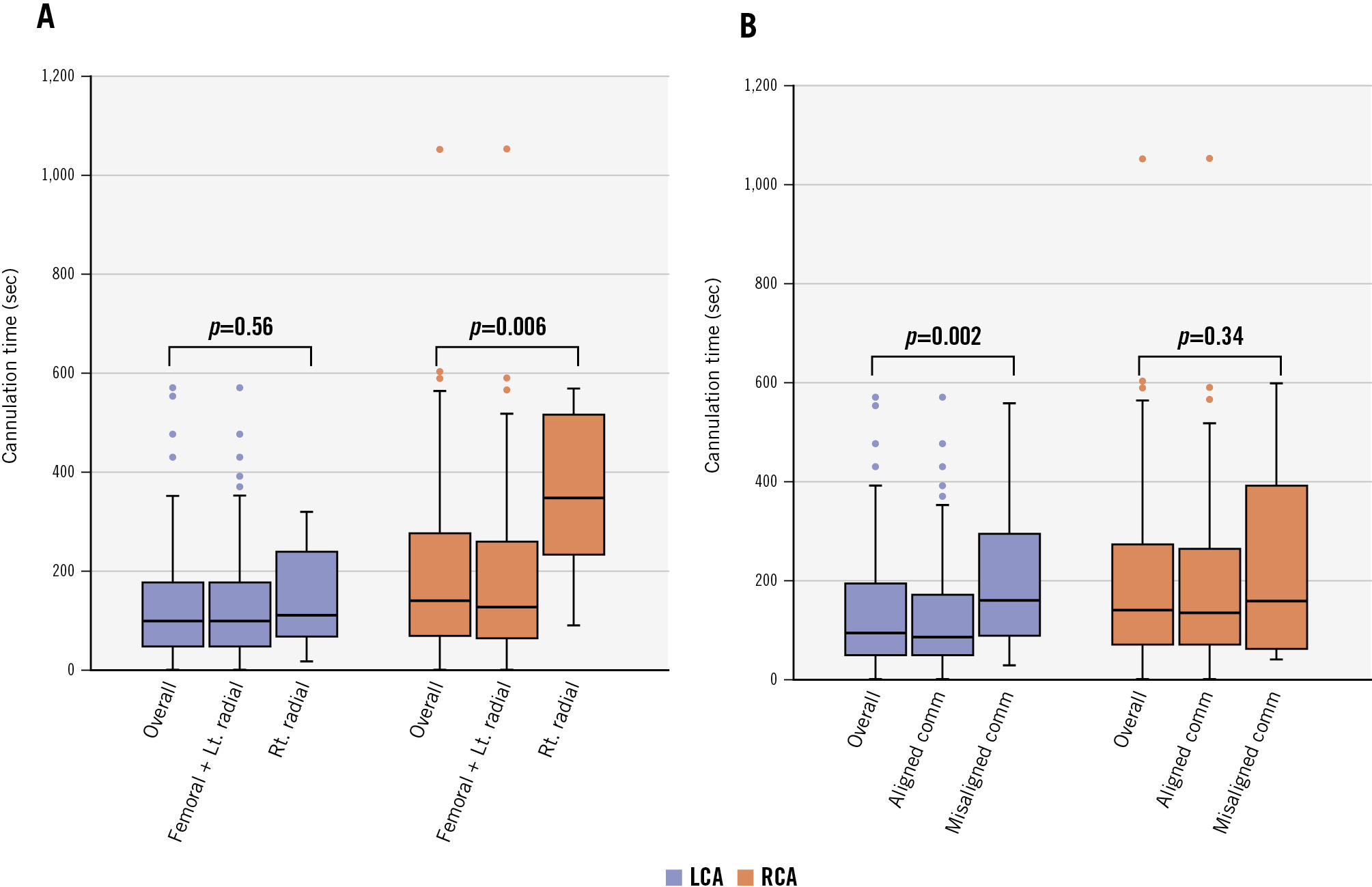
Figure 2. Coronary cannulation time based on access site and commissural alignment. The median times of left and right coronary cannulation are shown, based on (A) access site and (B) commissural alignment. Upper whisker: maximum value to quartile 3; lower whisker: quartile 1 to minimum value; box: interquartile range; central line: median. Comm: commissure; LCA: left coronary artery; Lt.: left; RCA: right coronary artery; Rt.: right
Commissural alignment
Procedural characteristics based on commissural alignment are depicted in Table 2. Although the selective/non-selective rate for LCA cannulation was comparable in the aligned versus misaligned groups, the former required a significantly shorter cannulation time compared to the latter (p=0.002). As for RCA cannulation, a significant number of patients had less selective and more frequent failed cannulation in the misaligned group (p=0.003); however, the cannulation time did not differ between the groups (Figure 2B).
Coronary alignment
Procedural characteristics based on coronary alignment are reported in Table 3. Patients who achieved coronary alignment showed significantly shorter cannulation times compared to the misaligned group for both the LCA (p=0.02) and the RCA (p=0.04) (Central illustration). The selective/non-selective rate for LCA cannulation was comparable in the aligned and misaligned coronary groups, whereas a significant number of patients had less selective and more frequent failed cannulation in the misaligned group (p=0.0007) for RCA cannulation.
Table 3. Procedural characteristics based on coronary alignment.
| LCA coronary alignment | RCA coronary alignment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Procedural characteristics | N | Overall | Aligned | Misaligned | p-value | N | Overall | Aligned | Misaligned | p-value |
| N | 126 | 126 | 100 | 26 | 126 | 126 | 98 | 28 | ||
| Evolut FX size | 126 | 0.0002 | 126 | 0.49 | ||||||
| 23 mm | 15 | 6 (6) | 9 (35) | 15 | 12 (12) | 3 (11) | ||||
| 26 mm | 31 | 26 (26) | 5 (19) | 31 | 21 (21) | 10 (36) | ||||
| 29 mm | 49 | 38 (38) | 11 (42) | 49 | 40 (41) | 9 (32) | ||||
| 34 mm | 31 | 30 (30) | 1 (4) | 31 | 25 (26) | 6 (21) | ||||
| NCC depth, mm | 126 | 3 [3-3] | 3 [3-3] | 3 [3-5] | 0.02 | 126 | 3 [3-3] | 3 [3-3] | 3 [2-4] | 0.62 |
| LCC depth, mm | 126 | 4 [3-6] | 4 [3-6] | 4 [4-7] | 0.71 | 126 | 4 [3-6] | 4 [3-6] | 4 [4-6] | 0.48 |
| THV/annular oversizing by perimeter, % | 126 | 17.2 [13.1-21.4] | 18.1 [13.3-22.7] | 15.0 [12.7-18.9] | 0.05 | 126 | 17.2 [13.1-21.4] | 17 [13.4-21.4] | 18.2 12.2-21.4] | 0.86 |
| THV/SoV relation*, % | 126 | –7.5 [–13.4 to –3.2] | –6.8 [–13.3 to –2.8] | –10.2 [–14.3 to –4.9] | 0.186 | 126 | –7.5 [–13.4 to –3.2] | –8 [–13.4 to –3.3] | –6.1 [–10.7 to –2.8] | 0.21 |
| THV/SoV relation**, % | 126 | –26.5 [–31.3 to –23.0] | –26.9 [–31.5 to –23.3] | –24.6 [–27.8 to –22.0] | 0.0497 | 126 | –26.5 [–31.3 to –23.0] | –26.9 [–31.6 to –23.3] | –24.8 [–27.3 to –22.0] | 0.049 |
| Coronary cannulation† | 126 | 126 | 100 | 26 | 0.92 | 122 | 122 | 96 | 26 | 0.0007 |
| Selective | 93 | 74 (74) | 19 (73) | 77 | 66 (68) | 11 (42) | ||||
| Non-selective | 33 | 26 (26) | 7 (27) | 41 | 31 (32) | 11 (42) | ||||
| Failure | 0 | 0 | 0 | 4 | 0 | 4 (16) | ||||
| Coronary cannulation time†, sec | 126 | 99 [48-175] | 83 [48-165] | 152 [65-304] | 0.02 | 122 | 140 [70-290] | 129 [72-255] | 243 [85-508] | 0.04 |
| Coronary cannulation access† | 126 | 126 | 100 | 26 | 0.76 | 126 | 126 | 98 | 28 | 0.63 |
| Via femoral | 95 | 76 | 19 | 95 | 77 | 18 | ||||
| Via radial | 31 | 24 | 7 | 31 | 21 | 10 | ||||
| Neo-comm-comm angle, degrees | 126 | 13 [6-22] | 10 [5-17] | 30 [17-41] | <0.001 | 126 | 13 [6-22] | 11 [5-18] | 30 [10-41] | <0.001 |
| Commissural misalignment | 126 | 126 | 100 | 26 | <0.001 | 126 | 126 | 98 | 28 | <0.001 |
| None | 77 | 72 (72) | 5 (19) | 77 | 66 (67) | 11 (39) | ||||
| Mild | 32 | 21 (21) | 11 (42) | 32 | 28 (29) | 4 (14) | ||||
| Moderate | 14 | 7 (7) | 7 (27) | 14 | 4 (4) | 10 (36) | ||||
| Severe | 3 | 0 | 3 (12) | 3 | 0 | 3 (11) | ||||
| Neo-comm-CA angle†, degrees | 126 | 46 [34-53] | 50 [42-55] | 20 [16-25] | <0.001 | 126 | 44 [34-52] | 48 [40-54] | 20 [14-28] | <0.001 |
| Coronary misalignment†† | 126 | 126 | 100 | 26 | <0.001 | 126 | 126 | 98 | 28 | <0.001 |
| None | 64 | 64 (57) | 0 | 61 | 61 (62) | 0 | ||||
| Mild | 36 | 36 (28) | 0 | 37 | 37 (38) | 0 | ||||
| Moderate | 21 | 0 | 21 (81) | 20 | 0 | 20 (71) | ||||
| Severe | 5 | 0 | 5 (19) | 8 | 0 | 8 (29) | ||||
| STJ-THV distance, mm | 126 | 2.5 [1.9-3.6] | 2.8 [2-3.9] | 2.2 [1.7-3] | 0.048 | 126 | 2.5 [1.9-3.6] | 2.5 [2-3.8] | 2.6 [1.6-3.5] | 0.45 |
| Values are n, n (%), or median [interquartile range]. *Calculated using the label size diameter provided by the THV manufacturer. **Calculated using the waist diameter provided by the THV manufacturer. †Corresponds to LCA cannulation for LCA coronary alignment and RCA cannulation for RCA coronary alignment. ††Corresponds to LCA misalignment for LCA coronary alignment and RCA misalignment for RCA coronary alignment. CA: coronary artery; comm: commissure; LCA: left coronary artery; LCC: left coronary cusp; NCC: non-coronary cusp; RCA: right coronary artery; SoV: sinus of Valsalva; STJ: sinotubular junction; THV: transcatheter heart valve | ||||||||||
Predictors of suboptimal CC after TAVI with Evolut FX
The main predictors of failed/delayed coronary cannulation after TAVI with Evolut FX are shown in Table 4 and the Central illustration. On multivariable analysis, LCA height (adjusted OR 0.73, 95% CI: 0.57-0.90; p=0.006) and coronary misalignment (adjusted OR 4.58, 95% CI: 1.45-14.47; p=0.009) were associated with failed/delayed LCA cannulation. Predictors of suboptimal CC for the RCA were sinus of Valsalva width (adjusted OR 0.63, 95% CI: 0.44-0.90; p=0.007) and coronary misalignment (adjusted OR 4.64, 95% CI: 1.29-16.74; p=0.019). Representative cases of favourable and unfavourable RCA cannulation with post-TAVI CT are shown in Figure 3.
Table 4. Univariate and multivariable logistic regression analyses of factors associated with suboptimal coronary cannulation
| Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|
| Crude OR | 95% CI | p-value | Adjusted OR | 95% CI | p-value | |
| LCA | ||||||
| LCA height, mm | 0.75 | 0.62-0.88 | 0.001 | 0.73 | 0.57-0.90 | 0.006 |
| LCC width, mm | 0.87 | 0.77-0.97 | 0.015 | 1.12 | 0.81-1.54 | 0.489 |
| Commissural misalignment | 6.34 | 2.18-20.19 | 0.001 | 3.77 | 0.99-14.24 | 0.051 |
| LCA coronary misalignment | 6.65 | 2.66-17.50 | <0.001 | 4.58 | 1.45-14.47 | 0.009 |
| Radial approach | 0.76 | 0.27-1.29 | 0.572 | 0.52 | 0.15-1.82 | 0.305 |
| Valve depth <3 mm (LCC) | 2.07 | 0.81-6.03 | 0.135 | 1.35 | 0.42-4.35 | 0.614 |
| STJ-THV distance, mm | 0.81 | 0.58-1.11 | 0.193 | 0.87 | 0.49-1.48 | 0.620 |
| THV/annular oversizing, % | 0.97 | 0.90-1.03 | 0.280 | 0.99 | 0.91-1.08 | 0.855 |
| THV/SoV relation*, % | 1.09 | 1.02-1.18 | 0.015 | 1.07 | 0.91-1.27 | 0.402 |
| RCA | ||||||
| RCA height, mm | 0.88 | 0.77-0.99 | 0.030 | 0.96 | 0.83-1.12 | 0.644 |
| RCC width, mm | 0.77 | 0.66-0.89 | <0.001 | 0.63 | 0.44-0.90 | 0.007 |
| Commissural misalignment | 4.19 | 1.45-12.43 | 0.008 | 1.70 | 0.37-7.81 | 0.498 |
| RCA coronary misalignment | 4.28 | 1.70-10.94 | 0.002 | 4.64 | 1.29-16.74 | 0.019 |
| Radial approach | 1.90 | 0.74-4.71 | 0.173 | 1.67 | 0.48-5.78 | 0.421 |
| Valve depth <3 mm (NCC) | 1.45 | 0.55-4.30 | 0.460 | 2.79 | 0.80-9.78 | 0.109 |
| STJ-THV distance, mm | 0.65 | 0.42-0.93 | 0.019 | 0.98 | 0.55-1.74 | 0.947 |
| THV/annular oversizing, % | 0.96 | 0.89-1.03 | 0.234 | 1.02 | 0.93-1.11 | 0.705 |
| THV/SoV relation*, % | 1.09 | 1.01-1.18 | 0.021 | 0.86 | 0.72-1.02 | 0.074 |
| *Calculated using the waist diameter provided by the THV manufacturer. CI: confidence interval; LCA: left coronary artery; LCC: left coronary cusp; NCC: non-coronary cusp; OR: odds ratio; RCA: right coronary artery; RCC: right coronary cusp; SoV: sinus of Valsalva; STJ: sinotubular junction; THV: transcatheter heart valve | ||||||
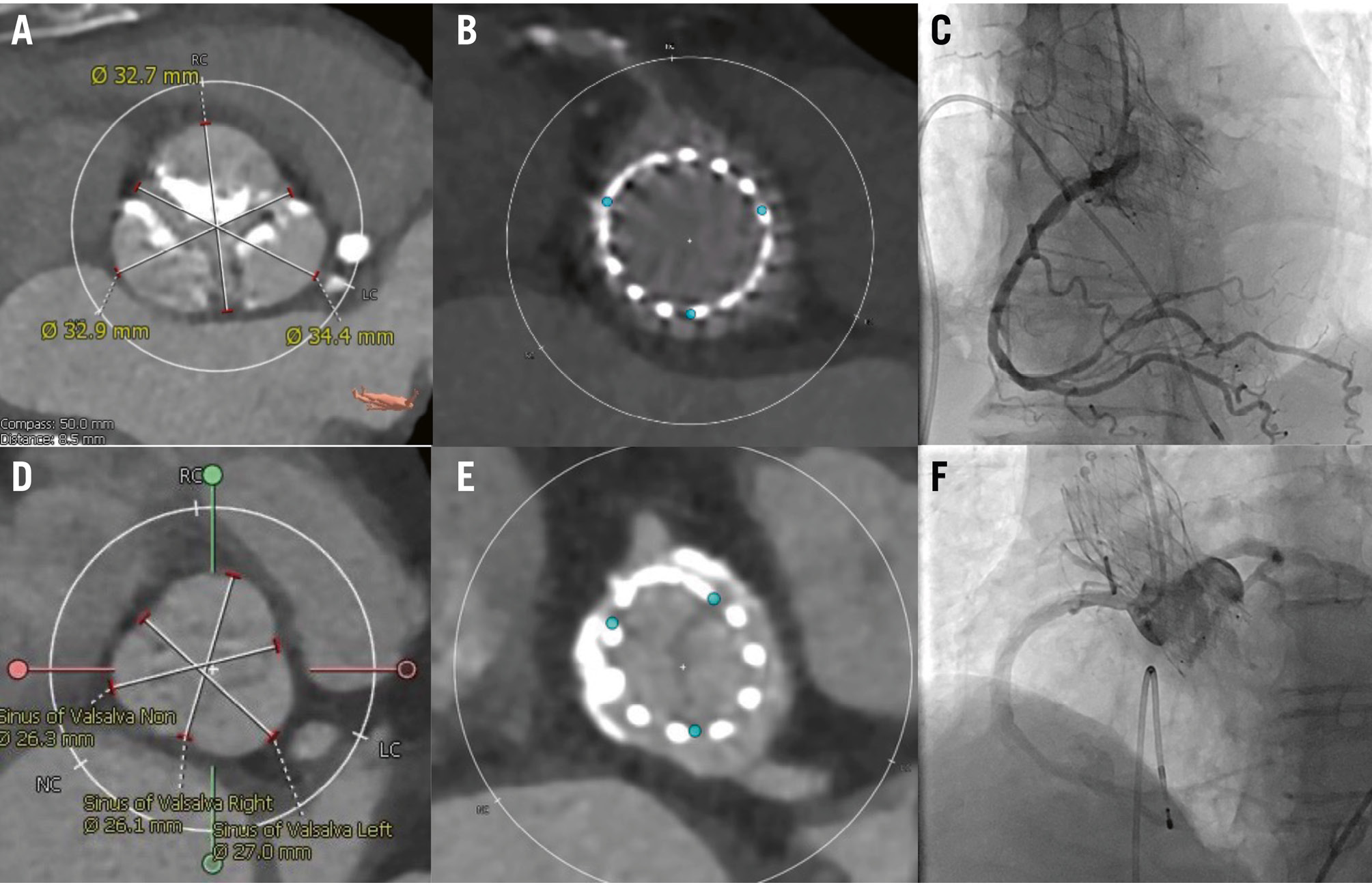 Figure 3. Post-TAVI CT showing favourable and unfavourable RCA cannulation. This figure shows post-TAVI CT which shows favourable (A-C) and unfavourable (D-F) anatomy for RCA cannulation. A) Wide sinus of Valsalva, (B) space between the Evolut FX 29 mm and the coronary ostium, (C) selective coronary cannulation achieved. D) Narrow sinus of Valsalva, (E) limited space between the Evolut FX 26 mm and the coronary ostium, (F) non-selective coronary cannulation. CT: computed tomography; RCA: right coronary artery; TAVI: transcatheter aortic valve implantation
Figure 3. Post-TAVI CT showing favourable and unfavourable RCA cannulation. This figure shows post-TAVI CT which shows favourable (A-C) and unfavourable (D-F) anatomy for RCA cannulation. A) Wide sinus of Valsalva, (B) space between the Evolut FX 29 mm and the coronary ostium, (C) selective coronary cannulation achieved. D) Narrow sinus of Valsalva, (E) limited space between the Evolut FX 26 mm and the coronary ostium, (F) non-selective coronary cannulation. CT: computed tomography; RCA: right coronary artery; TAVI: transcatheter aortic valve implantation
Discussion
This study represents the first multicentre dataset to demonstrate the impact of both commissural and coronary alignment on CC, as confirmed by post-TAVI CT, after Evolut FX implantation. Our main findings are as follows: (1) CC after TAVI with the Evolut FX was successfully achieved in the majority of our patients; (2) most individuals who underwent TAVI with the Evolut FX achieved commissural and coronary alignment; (3) significantly longer cannulation times were required in misaligned left and right coronary arteries, compared to those that were aligned; (4) the type of coronary access site (femoral/left radial vs right radial) did not impact successful cannulation rates, but RCA cannulation via right radial access required a longer cannulation time than via femoral/left radial access; and (5) predictors associated with suboptimal LCA cannulation were LCA height and misaligned LCA, whereas factors associated with suboptimal RCA cannulation were RCC width and misaligned RCA. The recommended age for indication of TAVI varies among guidelines111213, but a recent study showed that a significant number of cases have been performed in younger patients, even below the age of 65 in the United States14. These patients have longer life expectancy; therefore, future coronary events and a need for CC could be anticipated. Previous studies have reported challenges with coronary access in patients with supra-annular THVs12. The ALIGN-ACCESS study was the first to demonstrate the association of commissural misalignment and impaired coronary access in patients who received supra-annular self-expanding THVs2. However, only fluoroscopic assessment was utilised in that study, and no information was obtained regarding coronary alignment, since post-TAVI CT was not performed. Moreover, only prior iterations, the Evolut R and PRO, were included for the CoreValve platform (all Medtronic). Our CANNULATE TAVR EXPANDED study is the first multicentre evaluation to show the association of both commissural and coronary alignment with the feasibility of CC using the Evolut FX. Thus, we were able to provide new insights from this study. First, we only included cases with the new generation of the supra-annular, self-expanding Evolut FX which has 3 markers located 3 mm from the distal edge of the stent frame, designed to align with the valve commissures. This new feature could facilitate achieving “angiography-guided” commissural alignment, which ultimately helps to make CC easier69. Indeed, we were able to demonstrate a high commissural alignment rate, which potentially led to the high successful CC rate for both the LCA (100%) and the RCA (96.7%). Although we did not retrieve the delivery catheter from the ascending aorta to the descending aorta in order to adjust the alignment of the valve, a 3-marker-based further adjustment could potentially decrease the rate of commissural and coronary misalignment. Future investigation using CT simulation might guide us to close the current gap. The RE-ACCESS 2 study, a similar post-TAVI CC study but using older-generation THVs (Evolut R/PRO/PRO+ [Medtronic]) showed a higher rate of unsuccessful cannulation, compared to our study, for both the LCA (2.5%) and the RCA (6.3%)15. Several factors are considered to be associated with the CC rate after TAVI, e.g., the type of THV, operator skill and experience, the study period, etc.; however, we assume that the above difference was mainly due to the difference between the Evolut FX and older-generation Evolut platforms. We experienced 4 cases of failed cannulation for the RCA (3.3%). Among them, coronary misalignment was observed in all cases, commissural misalignment in 75% (3/4), and a bicuspid aortic valve in 50% (2/4). Cusp asymmetry and coronary ostia eccentricity are frequently observed in patients with a bicuspid aortic valve16, so it may have impacted CC in our study; however, only 6 patients (4.8%) with a bicuspid aortic valve were included in this study, so further prospective study is warranted in this specific population. Second, differently from the previous studies, we performed post-TAVI CT assessment in all patients, which provided additional insights. The majority of patients who underwent TAVI with the Evolut FX in our series achieved commissural and coronary alignment, which was in line with our initial reports69. Additionally, with detailed analysis of coronary alignment using post-TAVI CT, we were able to demonstrate for the first time that coronary misalignment was strongly associated with suboptimal CC for both the LCA (OR 4.58) and the RCA (OR 4.64). Coronary misalignment remained significant even after adjusting for other considerable factors such as coronary height, the size of the sinus of Valsalva, STJ-THV distance, commissural alignment, access site, implanted THV depth, THV/annular oversizing, and THV/SoV relation. In our study, THV depth was not associated with suboptimal cannulation, in line with the RE-ACCESS 2 study15. The reasons to explain this are as follows: (1) the optimal implantation depth is achieved (LCC depth 4 [IQR 3-6] mm, NCC depth 3 [IQR 3-3] mm) in the majority of the cases, guided by 3 markers located 3 mm from the distal stent frame, and the operators potentially adjust the depth based on patients’ anatomy (e.g., implanting slightly deeper in patients with relatively low coronary takeoff); and (2) the impact of coronary alignment is much higher than that of the implantation depth, since the non-accessible stent frame can be as high as 26 mm (from the distal stent frame, in case of a severe coronary misalignment case) to as low as 13 mm (from the distal stent frame, in case of optimal coronary alignment). As for the access site, previous studies have all assessed CC feasibility only from the femoral artery1215. Although a limited number of patients (N=31) received CC from the radial artery in our study, we were able to capture some important findings. We combined the results of the left radial and femoral approaches due to the similarity of their catheter trajectories. The success rate for LCA cannulation and cannulation time via the right radial artery were comparable to those via the femoral/left radial artery approach, whereas for RCA cannulation, the success rate was comparable between the two groups. However, cannulation time via the right radial artery was significantly longer than the time via the femoral/left radial artery approach. The presence of a tortuous brachiocephalic artery can limit the subtle manipulation of the catheter which is required to engage the RCA, and it can be more prominent in case of a horizontal aorta. Whether this finding is also relevant in patients undergoing percutaneous coronary intervention (PCI) warrants further investigation. Cannulation of the RCA is usually considered to be more difficult than that of the LCA. In our study, the size of the SoV and RCA misalignment were identified as predictors of suboptimal cannulation. If the patient has a wider SoV, there is less chance of suboptimal cannulation (OR 0.63). This is in line with previous studies showing a high THV/SoV relation is associated with impaired coronary access12. In case of a small SoV relative to THV size, the free space between the THV frame and the coronary ostia is limited; therefore, it makes it difficult for operators to freely manipulate the catheters, which negatively impacts CC. The newest-iteration − Evolut FX+ (Medtronic) − was designed to improve coronary access. Its frame features 3 large windows which are the size of 4 normal frame cells and positioned 120 degrees apart between the commissures without affecting the radial strength or valve performance. It is considered to facilitate rapid coronary access; however, its advantage may be attenuated if the valve is implanted with coronary misalignment. Whether implantation of this latest platform improves the coronary cannulation success rate and shortens cannulation time warrants further evaluation. A previous study has shown the impact of commissural alignment on valve performance17. In that study, haemodynamic performance indices such as mean pressure gradient and effective orifice index were not significantly different between patients with commissural alignment and misalignment. No transvalvular aortic regurgitation was identified in that series. However, the relatively small sample size precludes definitive conclusions, and a larger-scale study is warranted to investigate the impact of commissural alignment on valve performance.
Limitations
Although this study was conducted as a multicentre study, the operators were highly skilled and experienced in both structural and coronary interventions; therefore, these findings might not be reproducible in every centre. The relatively small sample size of this study might have affected the results, especially the comparison between CC via the femoral versus the radial artery; however, this was the first study to assess CC feasibility from the radial artery post-TAVI. While larger, multicentre studies are required to confirm our results, it is important to emphasise that the prospective nature associated with the inclusion of consecutive patients and consistent performance of CT post-TAVI allowed for interesting insights on this important topic. Although all the CT analyses performed in this study were evaluated by experienced interventional imagers in each large-scale hospital, it should be acknowledged that the CT analyses in this study were not reviewed by an independent core laboratory. The success rate of CC could be higher in the setting of PCI which allows the utilisation of a coronary guidewire, balloon, and guide extension catheter, which can facilitate coronary access.
Conclusions
Coronary cannulation after TAVI with the new-generation Evolut FX was highly feasible when implanted using best contemporary practice due to a high rate of commissural and coronary alignment. Significantly longer cannulation times were required for both misaligned left and right coronary arteries. Coronary misalignment was associated with suboptimal CC for both the LCA and the RCA.
Impact on daily practice
Coronary cannulation after transcatheter aortic valve implantation (TAVI) with tall-frame, supra-annular transcatheter heart valves (THVs) has been reported to be challenging. In this study, we demonstrated that coronary cannulation after TAVI with the new-generation Evolut FX – implanted using best contemporary practice – was highly feasible due to a high achievement rate of commissural and coronary alignment. We furthermore identified coronary misalignment as a strong predictor for suboptimal coronary cannulation of both the left coronary artery and the right coronary artery. Larger multicentre studies are required to confirm whether a newer-generation THV platform can further improve coronary access.
Acknowledgements
The authors thank the investigators and institutions that have participated in the CANNULATE TAVR EXPANDED study.
Conflict of interest statement
Y. Ohno is a clinical proctor for Medtronic and Abbott. N. Kamioka is a clinical proctor for Edwards Lifesciences. G.F. Attizzani is a consultant, serves on the advisory board, and has research grants with Medtronic, Boston Scientific, and Dasi Simulations. A. Ukaigwe is a consultant for Medtronic. S. Filby is a consultant for Boston Scientific. The other authors have no conflicts of interest relevant to the contents of this paper to declare.
Supplementary data
To read the full content of this article, please download the PDF.

